MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
ELECTROLYTE
ANODE Product
CATHODE Product
(aq) with electrode

Important Questions on Electrochemistry
HARD
JEE Main/Advance
IMPORTANT
| ELECTROLYTE | ANODE Product | CATHODE Product |
| with Inert electrode |
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Identify the titration curve for equimolar mixture of and titrated with .
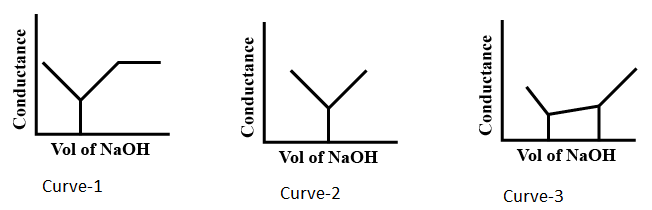
If your answer is first curve, write the answer as Curve-1.
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
