Describe the changes to the kinetic energy, the potential energy and the total internal energy of its molecules as ice melts into water.
Ice melts at .
Describe the changes to the kinetic energy, the potential energy and the total internal energy of its molecules as ice melts into water.
Ice melts at .

Important Questions on Thermal Physics
Describe the changes to the kinetic energy, the potential energy and the total internal energy of the molecules of a block of ice as:
The temperature of the water rises from to room temperature.
The so-called 'zeroth law of thermodynamics' states that if the temperature of body is equal to the temperature of body and
the temperature of body is the same as body , then the temperature of body equals the temperature of body
Explain, in terms of energy flow, why the concept of temperature would be meaningless if this law was not obeyed.
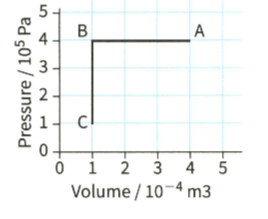
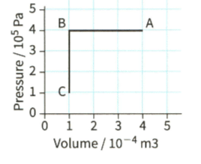
. Figure shows a fixed mass of gas that undergoes a change from to and then to
During the change from toof thermal energy (heat) is removed from the gas. Calculate the change in the internal energy of the gas.
Figure shows a fixed mass of gas that undergoes a change from to and then to
During the change from to C, the internal energy of the gas decreases by . Using the first law of thermodynamics explain how this change can occur.
When a thermocouple has one junction in melting ice and the other junction in boiling water it produces an e.m.f. of .
What e.m.f. would be produced if the second junction was also placed in melting ice?