MEDIUM
JEE Main
IMPORTANT
Earn 100
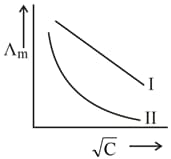
Above plot represents the variation of molar conductivity against (where, molar concentration of the electrolyte). Select the incorrect options among the following.

(a)Both and curves are for strong electrolyte.
(b)Both and curves are for weak electrolyte.
(c)Curve is for strong electrolyte and curve is for weak electrolyte.
(d)Curve is for weak electrolyte and curve is for strong electrolyte.
50% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main
IMPORTANT
An aqueous solution of copper sulphate is electrolysed using platinum electrodes in one case and copper electrodes in another case. Will the products of electrolysis be the same or different. Give reason?
EASY
JEE Main
IMPORTANT
State the products of electrolysis obtained on the cathode and the anode in the following case:
A dilute solution of with platinum electrodes.
EASY
JEE Main
IMPORTANT
State the products of electrolysis obtained on the cathode and the anode in the following case:
An aqueous solution of with silver electrodes.
MEDIUM
JEE Main
IMPORTANT
A current of Ampere is passed for one hour between nickel electrodes in of solution. What will be the molarity of the solution at the end of the electrolysis?
EASY
JEE Main
IMPORTANT
On electrolysis of an aqueous solution of , why is and not liberated at the cathode?
MEDIUM
JEE Main
IMPORTANT
Which of the following gives the charge of an electron?
HARD
JEE Main
IMPORTANT
In the electrolysis of copper chloride solution, the mass of cathode is increased by . At the copper anode, we will have
MEDIUM
JEE Main
IMPORTANT
Which of the following statements are incorrect?
