EASY
Earn 100
Does acetic acid give iodoform test?
Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
Columns 1, 2 and 3 contain starting materials, reaction conditions and type of reactions respectively.
| Column 1 | Column 2 | Column 3 |
| (I) Toulene | (i) | (P) Condensation |
| (II) Acetophenone | (ii) | (Q) Carboxylation |
| (III) Benzaldehyde | (iii) | (R) Substitution |
| (IV) Phenol | (iv) | (S) Haloform |
HARD
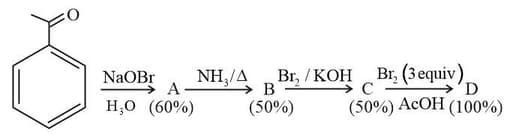
In the following reaction sequence, the amount of (in g) formed from moles of acetophenone is ____.
(Atomic weights in The yield (%) corresponding to the product in each step is given in the parenthesis)
EASY
Give one chemical test each to distinguish between acetaldehyde and benzaldehyde.
HARD
An organic compound gives red precipitate when warmed with Fehling’s solution. Give the IUPAC name of the compound and write the chemical equation for the reaction.
EASY
Which of the following pairs of organic compounds give positive Tollen's test?
EASY
How will you distinguish between propanal and propanone?
EASY
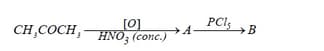
Identify the compounds A and B in the given reactions:
EASY
Write the chemical equations for the following reaction: Haloform reaction
HARD
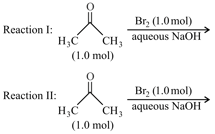
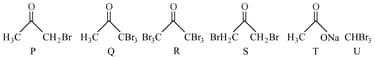
After completion of the reactions (I and II), the organic compound(s) in the reaction mixtures is(are):
MEDIUM
The major products formed in the following reaction sequence and are:
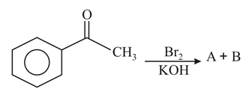
MEDIUM
The reaction of ethyl methyl ketone with excess gives the following major product
MEDIUM
The compound that does not undergo haloform reaction is
EASY
The compound that reduces Tollens' reagent is
EASY
Name one reagent used to distinguish acetaldehyde from acetone.
EASY
What happens when (Write chemical equations only) Acetone reacts with iodine in presence of ?
MEDIUM
In the following, which one reduces Fehling solution -
MEDIUM
How will you distinguish between Aldehyde and Ketone by chemical test?
MEDIUM
The structure of the starting compound used in the reaction given below is:
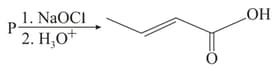
MEDIUM
Haloform reaction with and will be responded by
EASY
Which is the most suitable reagent for the following conversion?


