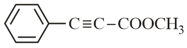Find the hybridization of
Important Questions on Chemical Bonding and Molecular Structure
In the following passage there are blanks, each of which has been numbered. There are questions given below the passage and for each question, four words are suggested, one of which fits the blank appropriately. Find out the appropriate word in each case.
Whenever I go into a bank, I feel scared. Everybody and everything that I see there (1) ____ me. As for the manager the sight (2) ____ him simply terrifies me and (3) ____ me want to runaway (4) _____ I can. As soon as I (5) ____ the door of the bank I lose my head (6) ____ when I try to do any (7) ____ there, I behave like an idiot. I cannot explain (8) ____ for this but that is how it (9) ____ has been that is how it is (10) ____.
Select the most appropriate option for blank No. 10.
(i)
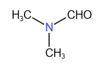 (ii)
(ii) 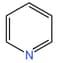
(iii) (iv)
Draw the shape of the following molecule:
| Column I | Column II |
| a. | (i) T-shape |
| b. | (ii) Pentagonal bipyramidal |
| c. | (iii) Linear |
| d. | (iv) Square – pyramidal |
| (v) Tetrahedral |
Draw the shape of the following molecule:
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?