EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
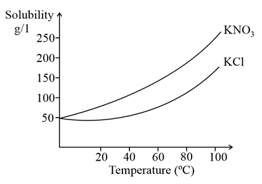
Given the solubility curves of and , which of the following statements is not true?

(a)At room temperature the solubility of and are not equal
(b)The solubilities of both and increase with temperature
(c)The solubility of decreases with temperature
(d)The solubility of increases much more as compared to that of with increase in temperature
37.5% studentsanswered this correctly

Important Questions on Ionic Equilibrium
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
If the of a mixture of of and of solution is , the value of is the closest to:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An aqueous solution of has a of . When water is added to increase the to , the hydrogen ion concentration is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The solubility product of is . Concentrated aqueous solution is added to a aqueous solution of . The at which precipitation occurs is:
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
The solubility curve of in water is shown below:
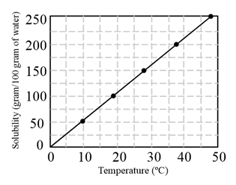
The amount of that dissolves in of water at is closest to:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
An aqueous buffer is prepared by adding of Acetic acid to of of Sodium acetate. If of acetic acid is , the of the buffer is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The of a weak acid is . The concentrations of the acid and its conjugate base are equal at a of:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The degree of dissociation of acetic acid in water ( of acetic acid is ) is:
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The of aqueous solutions of and will follow the order
