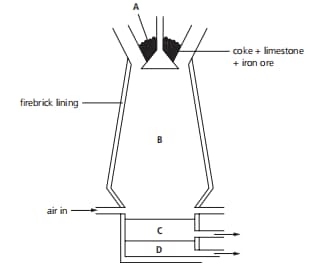
The diagram shows a blast furnace. Which one of the raw materials is added to the blast furnace to help remove the impurities from the iron ore?


Important Questions on Cambridge IGCSE Exam Questions from Paper 2
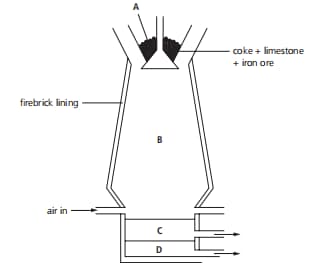
The diagram shows a blast furnace. The impurities are removed as a slag. Which letter on the diagram shows the slag?
Carbon monoxide is formed in the blast furnace by the reaction of coke with oxygen.
Complete the equation for this reaction.
Carbon monoxide is formed in the blast furnace by reaction of coke with oxygen.
State the adverse affect of carbon monoxide on human health.
In the hottest regions of the blast furnace, the following reaction takes place.
Which two of these five sentences correctly describe this reaction?
The iron oxide gets reduced.
The reaction is thermal decomposition.
The carbon gets oxidized.
The carbon gets reduced.
Carbon neutralizes the iron oxide.
Aluminium cannot be extracted from aluminium oxide in a blast furnace. Explain why aluminium cannot be extracted in this way.
State the name of the method used to extract aluminium from its oxide ore.
Calcium carbonate, , is the raw material used in the manufacture of lime, . Describe how lime is manufactured from calcium carbonate.
