The molar solubility (in ) of a sparingly soluble salt is ‘s’. The corresponding solubility product is ‘s’ is given in terms of by the relation :
The molar solubility (in ) of a sparingly soluble salt is ‘s’. The corresponding solubility product is ‘s’ is given in terms of by the relation :
Important Questions on Solutions

The gas with the highest value of Henry's law constant is
Henry's constant (in kbar) for four gases and in water at is given below :
(density of water at ) This table implies that :
The oxygen dissolved in water exerts a partial pressure of in the vapour above water. The molar solubility of oxygen in water is ______
(Round off to the Nearest Integer).
[Given : Henry's law constant for Density of water with dissolved oxygen]
gas is bubbled through water during a soft drink manufacturing process at . If exerts a partial pressure of then of would dissolve in of water. The value of is _______. (Nearest integer)
(Henry's law constant for at is )
[Henry's law constant for at bar; density of water at ; mass of ; molar mass of water ]
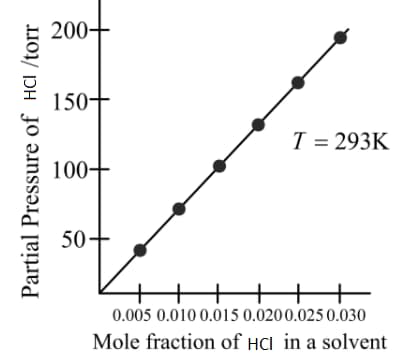
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is