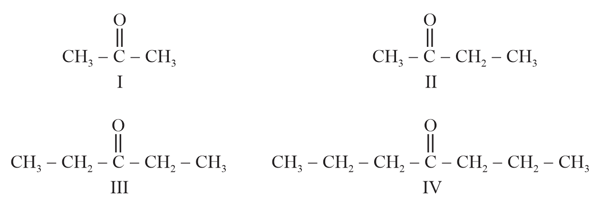What is the effects of alpha hydrogen in boiling point of ketone and aldehyde?
Important Questions on Aldehydes, Ketones and Carboxylic Acids
Given below are two statements:
Statement : The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statement : The boiling points of aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of -bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :
How would you account for:
Boiling points of aldehydes are lower than alcohols.
Arrange the following compounds in increasing order of their boiling points:
The odour from vanilla extract comes from the molecule vanillin.
Decreasing order of boiling point of to follow:
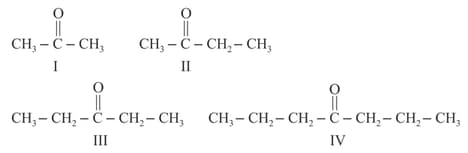
.
Arrange the following compounds in the increasing order of their boiling points.
Acetone, n-propyl alcohol, ethyl methyl ether and n-butane.
Which of the compound is soluble in ?
Decreasing order of boiling point of to follows: