MEDIUM
Earn 100
What type of bonding is there between hydrogen and fluorine in molecule ?
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
Give one example each of a liquid and a solid covalent bond.
HARD
Why are covalent compounds are generally poor conductors of electricity?
MEDIUM
Identify the correct electron dot structure of nitrogen molecule in the following:
EASY
Ethane with the molecular formula has
HARD
Give one word or a phrase for the following statement:
- The chemical bond formed by a shared pair of electrons each bonding atom contributing one electron to the pair.
MEDIUM
Explain how a single covalent bond is formed between two atoms of hydrogen. (Support your answer by drawing a structural diagram).
MEDIUM
Bonds present in are
EASY
What is a polar molecule? Give an example.
HARD
Draw the electron dot diagram for the compound given below. Represent the electrons by (.) and (x) in the diagram.
- Chlorine molecule [Atomic No.]
EASY
Ethane, with the molecular formula has
EASY
What do you understand by a lone pair of electrons?
MEDIUM
Which one of the following compounds is not an ionic compound?
MEDIUM
The number of covalent bonds present in pentane are
EASY
Which of the following options containing formula, bonding and nature of aqueous solution respectively is correct for the compound formed by two elements A and B having atomic numbers 1 and 17, respectively?
MEDIUM
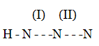
The above compound represents hydrogen azide, the bond orders of bonds and are:
MEDIUM
The number of coordinative covalent bonds in the structure of nitric acid is
EASY
Draw the electron dot structure of Nitrogen.
MEDIUM
A compound with a low boiling point is:
EASY
Which compound has both covalent as well as co-ordinate bonds?

