MEDIUM
JEE Advanced
IMPORTANT
Earn 100
(a) What is the average translational kinetic energy of a molecule of an ideal gas at a temperature of ?
(b) What is the total random translational kinetic energy of the molecules in one mole of this gas?
(c) What is the RMS speed of oxygen molecules at this temperature?

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
EASY
JEE Advanced
IMPORTANT
At and pressure, the densities of air, oxygen and nitrogen are , and , respectively. Calculate the percentage of nitrogen in the air from these data, assuming only these two gases to be present.
EASY
JEE Advanced
IMPORTANT
An air bubble of volume, is at the bottom of a lake deep where the temperature is . The bubble rises to the surface which is at a temperature of . Take the temperature to be the same as that of the surrounding water and find its volume just before it reaches the surface.
HARD
JEE Advanced
IMPORTANT
For a certain gas, the heat capacity at constant pressure is greater than that at constant volume by .
(a) How many moles of the gas are there?
(b) If the gas is monatomic, what are heat capacities at constant volume and pressure?
(c) If the gas molecules are diatomic which rotate but do not vibrate, what are heat capacities at constant volume and at constant pressure?
EASY
JEE Advanced
IMPORTANT
The heat capacity at constant volume of a sample of a monatomic gas is . Find
(a) the number of moles
(b) the internal energy at
(c) the molar heat capacity at constant pressure.
EASY
JEE Advanced
IMPORTANT
Two thermally insulated vessels and are filled with air at temperatures volumes and pressures , respectively. If the valve joining the two vessels is opened, the temperature inside the vessel at equilibrium will be (common pressure)
EASY
JEE Advanced
IMPORTANT
Two marks on a glass rod apart are found to increase their distance by when the rod is heated from to . A flask made of the same glass as that of rod, measures a volume of at . The volume it measures at in is
EASY
JEE Advanced
IMPORTANT
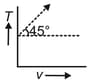
The given curve represents the variation of temperature as a function of volume for one mole of an ideal gas. Which of the following curves best represents the variation of pressure as a function of volume?
EASY
JEE Advanced
IMPORTANT
A gas is found to be obeyed the law constant. The initial temperature and volume are and . If the gas expands to a volume , then the final temperature becomes