MEDIUM
Earn 100
represents
(a)Change in amount of heat contained in a body as a result of temperature change
(b)Amount of heat energy which transits from one body to other due to temperature difference
(c)Both (Change in amount of heat contained in a body as a result of temperature change) and (Amount of heat energy which transits from one body to other due to temperature difference) are correct
(d)None of these
15.38% studentsanswered this correctly
Important Questions on Thermal Properties of Matter
EASY
Two moles of oxygen is mixed with eight moles of helium. The effective specific heat of the mixture at constant volume is
HARD
A water cooler of storage capacity litres can cool water at constant rate of . In a closed circulation system (as shown schematically in the figure), the water from the cooler is used to cool an external device that generates constantly of heat (thermal load). The temperature of water fed into the device can not exceed and the entire stored litres of water is initially cooled to . The entire system is thermally insulated. The minimum value of (in ) for which the device can be operated for hours is:
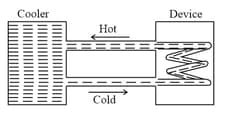
(Specific heat of water is and the density of water is )
HARD
A electrical heater is used to heat a container filled with of water. It is found that the temperature of the water and the container rise by in minutes. The container is then emptied, dried, and filled with of an oil. It is now observed that the same heater raises the temperature of the container-oil system by in minutes. Assuming no other heat losses in any of the processes, the specific heat capacity of the oil is
HARD
of ice at is mixed with of water at . Assuming that there is no loss of energy to the environment, what will be the final temperature of the mixture? (Assume latent heat of ice , specific heat of water and ice are and , respectively.)
HARD
A certain liquid has a melting point of and a boiling point of . A thermometer is designed with this liquid and its melting and boiling points are designated at and . The melting and boiling points of water on this scale are
HARD
A bullet whose specific heat is, and moving at, plunges into a block of wax whose specific heat is, . Both bullet and wax are at, and assume that the bullet comes to rest in the wax and all its kinetic energy goes into heating the wax. The thermal temperature of the wax in, is close to.
MEDIUM
An experiment takes to raise the temperature of water in a container from to and another to convert it totally into steam by a heater supplying heat at a uniform rate. Neglecting the specific heat of the container and taking specific heat of the water to be , the heat of vaporization according to this experiment will come out to be:
HARD
A current carrying wire heats a metal rod. The wire provides a constant power to the rod. The metal rod is enclosed in an insulated container. It is observed that the temperature in the metal rod changes with time as:
, where is a constant with appropriate dimension while is a constant with dimension of temperature. The heat capacity of the metal is:
EASY
The SI unit of mechanical equivalent of heat is
EASY
At what temperature do the Fahrenheit and Celcius scales of temperature coincide?
EASY
Ice is used in a cooler in order to cool its contents. Which of the following will speed up the cooling process?
MEDIUM
Solar energy is incident normally on the earth's surface at the rate of about . The distance between the earth and the sun is . Energy and mass are related by the Einstein equation, where is the speed of light in free space. The decrease in the mass of the sun is
MEDIUM
Water of volume 2 L in a closed container is heated with a coil of 1 kW. While water is heated, the container loses energy at a rate of 160 J/s. In how much time will the temperature of water rise from 27oC to 77oC ? (Specific heat of water is 4.2 kJ/kg and that of the container is negligible).
MEDIUM
Two different liquids of same mass are kept in two identical vessels, which are placed in a freezer that extracts heat from them at the same rate causing each liquid to transform into a solid. The schematic figure below shows the temperature T vs time t plot for the two materials. We denote the specific heat of materials in the liquid (solid) states to be and respectively.
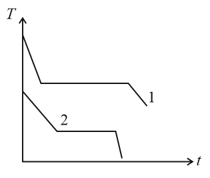
EASY
water is heated from to . Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water ):
EASY
The specific heat of water and the latent heat of ice . of ice at is placed in of water at . The amount of ice that will melt as the temperature of water reaches is close to (in grams)
EASY
A calorimeter of water equivalent contains of water at . ' grams of steam at is mixed in it till the temperature of the mixture is . The value of is close to (Latent heat of water, specific heat of water)
EASY
Pyrometer is a device for measuring
EASY
Mercury is often used in clinical thermometers. Which one of the following properties of mercury is not a reason for this?
MEDIUM
A thin paper cup filled with water does not catch fire when placed over a flame. This is because

