Use the first law of thermodynamics to answer the following.
The same gas is heated as before with of energy. This time the gas is allowed to expand so that it does of work on its surroundings. Calculate the change in the internal energy of the gas.

Important Questions on Thermal Physics
The electrical resistance of a pure copper wire is mostly due to the vibrations of the copper atoms. Table 19.1 shows how the resistance of a length of copper wire is found to change as it is heated. Copy the table and add a column showing the temperatures in. Draw a graph to show these data. (Start the temperature scale of your graph at ) Explain why you might expect the resistance of copper to be zero at this temperature.
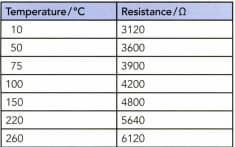
The variation of resistance with temperature for a length of copper wire.
Give one word for each of the following:
Adding a scale to a thermometer
Give one word for each of the following:
All the temperatures, from lowest to highest, which a thermometer can measure
Give one word for each of the following:
The extent to which equal rises in temperature give equal changes in the thermometer's output
How big a change in output is produced by a given change in temperature.
