MEDIUM
Earn 100
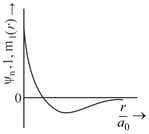
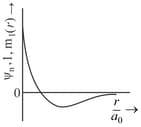
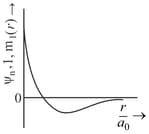
orbital has how many ______ nodal planes.
50% studentsanswered this correctly
Important Questions on Structure of Atom
MEDIUM
MEDIUM
MEDIUM
[Planck's constant ]
HARD
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
MEDIUM
HARD
| Column – 1 | Column – 2 | Column – 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite electron from state to state. |
HARD
EASY
MEDIUM
EASY
HARD
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P) |
| (II) orbital | (ii) One radial node | (Q) Probability density at the nucleus . |
| (III) orbital | (iii) | (R) Probability density is maximum at the nucleus. |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite an electron from state to state is times the energy needed to excite are electron from state to state. |
MEDIUM
EASY
The correct order of their increasing energies will be:
MEDIUM
MEDIUM
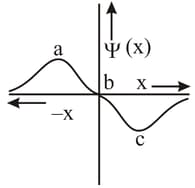
EASY
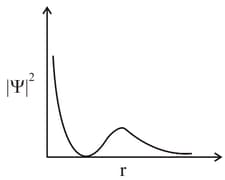
MEDIUM
EASY
MEDIUM
An electron in an orbital of high angular momentum stays away from the nucleus than an electron in the orbital of lower angular momentum.
For a given value of the principal quantum number, the size of the orbit is inversely proportional to the azimuthal quantum number.
According to wave mechanics, the ground state angular momentum is equal to .
The plot of Vs for various azimuthal quantum numbers, shows peak shifting towards higher value.
EASY