of copper powder was taken in a China dish and heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the colour of the products formed in each case.

Important Questions on Chemical Reactions and Equations
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct answer?
When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a :
Which of the following oxide(s) is/are soluble in water to form alkalies?
Study the diagram given below and identify the gas formed in the reaction.
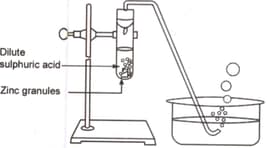
The above reaction is a/an example of
Which of the following statements about the reaction given below are correct?
(i) is oxidised to
(ii) is reduced to
(iii) acts as an oxidising agent
(iv) acts as an oxidising agent
