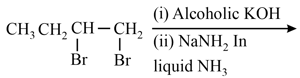EASY
Earn 100
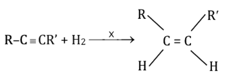
2-hexyne gives trans-2-hexene on treatment with –
(a)
(b)
(c)
(d)
100% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
EASY
HARD
 ?
?EASY
MEDIUM
Consider the following reactions:
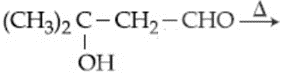
Which of the reaction(s) will not produce Saytzeff product?
EASY
MEDIUM
EASY
HARD
HARD
The major product in the following sequence of reaction is :
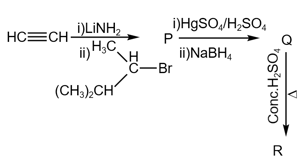
EASY
MEDIUM
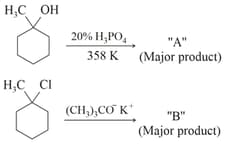
The product "" and "" formed in above reactions are
HARD
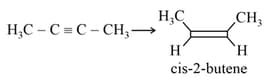
EASY
HARD