HARD
Earn 100
40 mL sample of 0.1 M solution of nitric acid is added to 20 mL of 0.3 M aqueous ammonia. What is the pH of the resulting solution?
(a)8.95
(b)89.5
(c)0.895
(d)4.48
50% studentsanswered this correctly
Important Questions on Equilibrium
EASY
HARD
MEDIUM
Which of the following mixtures will have the lowest pH at
EASY
MEDIUM
[Given:
MEDIUM
MEDIUM
HARD
[Given: of acetic acid molar mass of acetic acid , Neglect any changes in volume]
MEDIUM
MEDIUM
EASY
HARD
HARD
MEDIUM
EASY
EASY
HARD
A solution of weak base is titrated with of a strong acid . The variation of of the solution with the volume of added is shown in the figure below. What is the of the base? The neutralisation reaction is given by .
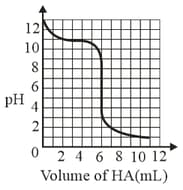
MEDIUM
EASY

