HARD
Earn 100
A solution contained and . of the solution required of HCl for
neutralization using phenolphthalein as an indicator. Addition of methyl orange required a further of the
acidic for neutralization. The amount of present in the solution is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Equilibria
HARD
HARD
EASY
HARD
HARD
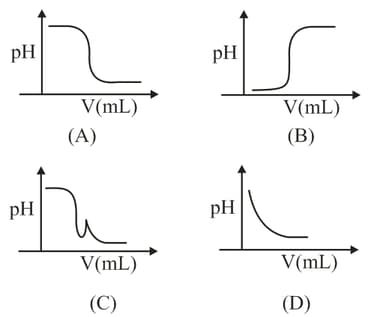
MEDIUM
HARD
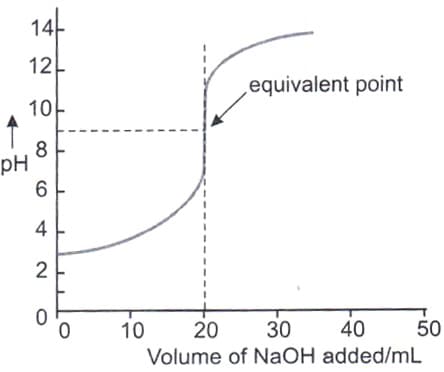
KIn value of phenolphthalein is 4.0 x 10-10. Thus, incorrect statement is
MEDIUM
HARD
EASY
HARD
MEDIUM
HARD
Phenolphthalein
HARD
EASY
Write the quadrants in which the following points lie:
HARD
EASY
MEDIUM
MEDIUM
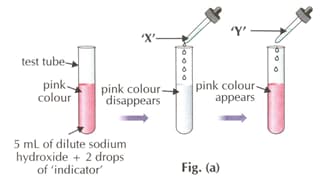
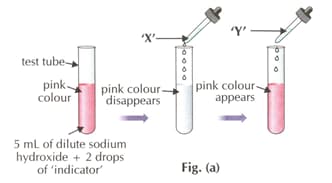
Which of the following indicators is used in the activity?
MEDIUM
What is substance 'Y'?