EASY
Earn 100
A Carnot engine converts of the available heat into work. Keeping the temperature of the reservoirs the same, two Carnot engines are used to replace the single Carnot engine: the heat ejected by the first is equal to the heat input to the second and the heat ejected by the second is to the lower reservoir. The efficiency of the second engine is (i.e. ). The efficiency of the first engine is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
EASY
A Carnot engine having an efficiency of is being used as a refrigerator. If the work done on the refrigerator is the amount of heat absorbed from the reservoir at a lower temperature is
EASY
A Carnot's engine having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is the amount of energy absorbed from the reservoir at lower temperature is
MEDIUM
A Carnot engine has an efficiency of When the temperature of the sink is reduced by , its efficiency is doubled. The temperatures of the source and the sink are, respectively,
HARD
A solid body of constant heat capacity is being heated by keeping it in contact with reservoirs in two ways: (i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies the same amount of heat. (ii) Sequentially keeping in contact with reservoirs such that each reservoir supplies the same amount of heat. In both, cases the body is brought from initial temperature to final temperature . Entropy change of the body in the two cases respectively is: Note: This question was awarded as a bonus since temperatures were given in centigrade instead of in Kelvin. Proper corrections are made in the question to avoid it.
EASY
Two carnot engines and are operated in series. The first one, receives heat at and rejects to a reservoir at temperature The second engine receives heat rejected by the first engine and, in turn, rejects to a heat reservoir at Calculate the temperature if the work outputs of the two engines are equal:
MEDIUM
A heat engine is involved with exchange of heat of and , during one cycle achieving and efficiency of . The value of is:
MEDIUM
A Carnot freezer takes heat from water at inside it and rejects it to the room at a temperature of The latent heat of ice is If 5kg of water at is converted into ice at by the freezer, then the energy consumed by the freezer is close to :
EASY
Two carnot engines and are connected in series in such a way that the work outputs are equal when the temperatures of hot and cold reservoirs of are and , and of engine are and , respectively. Then the temperature is
EASY
The coefficient of performance of a refrigerator is . If the temperature inside the freezer is , the temperature of the surrounding to which it rejects heat is:
EASY
A Carnot engine, having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is , the amount of energy absorbed from the reservoir at a lower temperature is:
EASY
The efficiency of a Carnot engine depends upon
EASY
The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
MEDIUM
A reservoir is at and Carnot's engine takes a thousand of heat from it and exhausts it to a sink at . What is the amount of work and the efficiency of the engine?
EASY
A refrigerator works between and . It is required to remove calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is:
(Take cal = Joules)
MEDIUM
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
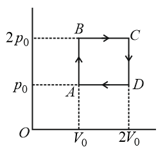
MEDIUM
Heat is flowing from a refrigerator whose inside temperature is , to a room at . Then the amount of heat delivered to the room for each joule of electrical energy consumed in joules is
EASY
The efficiency of carnot engine is when its hot and cold reservoirs are maintained at temperature and , respectively. To increase the efficiency to , the increase in temperature of the hot reservoir by keeping the cold one constant at is
EASY
The temperature inside a refrigerator is and the room temperature is . The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
MEDIUM
Three Carnot engines operate in series between a heat source at a temperature and a heat sink at temperature (see figure). There are two other reservoirs at temperature and as shown, with The three engines are equally efficient if:
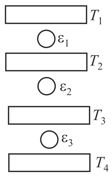
MEDIUM
A Carnot's engine works like a refrigerator between and . It receives heat from the reservoir at a lower temperature. The amount of work done in each cycle to operate the refrigerator is,

