MEDIUM
Earn 100
A Carnot engine used first ideal monoatomic gas and then an ideal diatomic gas, if the source and sink temperatures are and , respectively and the engine extract 1000 J of heat from the source in each cycle, then
(a)Area enclosed by the diagram is 10 J
(b)Heat energy rejected by engine is 1st case is 600 J while that in 2nd case in 113 J
(c)Area enclosed by the diagram is 500 J
(d)Efficiencies of the engine in both the cases are in ratio 21:25
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
EASY
HARD
EASY
(Take cal = Joules)
EASY
EASY
EASY
MEDIUM
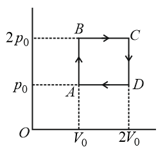
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
MEDIUM
EASY
EASY
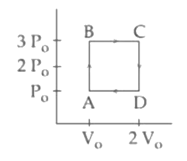
An engine operates by taking a monatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY

