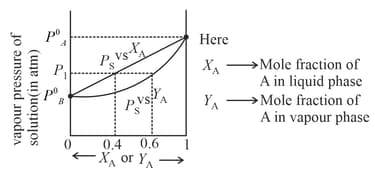
and are volatile liquid and form an ideal solution. Graph is given below in which upper dark line represent vapour pressure of solution and mole fraction of in liquid while lower dark line represent vapour pressure of solution and mole fraction of in vapour phase at temperature . Select the correct statement


Important Points to Remember in Chapter -1 - Solutions from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Solution:
A homogeneous mixture of two or more non-reacting substances is known as solution, Homogeneity or heterogeneity depends upon particle size and states of matter present in the solution. Every solution is made up of a solvent (present in larger quantity) and one or more solute (present in smaller quantity).
2. Units of Concentration:
(i) Molarity :
It is the number of moles of solute present per litre of solution.
millimoles
Molarity changes with temperature of the solution. Increase in temperature generally decreases the molarity. It is the most convenient method to express concentration of the solution. On dilution, molarity decreases.
(ii) Molality :
Number of moles of solute present per of solvent.
It is independent of temperature since no volume factor is involved in the equation.
(iii) Mole fraction :
It is the ratio of number of moles of one component to the total number of moles present in the solution.
For a system having two components and ,
Mole fraction is also independent of temperature.
(iv) In terms of
by weight
weight by volume (In case of solid dissolved in a liquid)
by volume (In case of liquid dissolved in another liquid)
by weight is independent of temperature while by volume are temperature dependent.
3. Henry’ s Law:
Solubility of a gas at a given temperature in a solvent is directly proportional to its partial pressure, if is the partial pressure of a gas and is its mole fraction in solution. Then , where is Henry's law constant for that gas.
4. Vapour Pressure and Raoult’s law:
The pressure exerted by the vapour at the free surface of liquid (provided system is closed) is known as its vapour pressure. The of a pure liquid is always greater than its solution (in case of non-volatile solute).
(i) Raoult’s Law for a solution having non-volatile solute.
Mole fraction of solute in solution
of pure solvent
of solution
i.e., relative lowering of vapour pressure is equal to the mole fraction of solute.
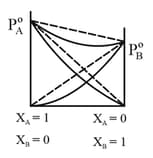
(ii) Raoult's Law of miscible liquid-liquid solution
For ideal solution, the partial vapour pressure is directly proportional to their mole fraction at constant temperature. For two components and in liquid solution.
The total pressure
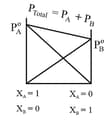
Most of the solutions show appreciable deviations from ideal behavior known as real or non-ideal solution. In some cases, the deviation is while in some cases deviation is .
5. Ideal and non-ideal Solutions:
The solutions which obey Raoult's law are ideal solutions and those which do not obey Raoult's law form non-ideal solution.
| Ideal Solution | Non-Ideal Solution | ||
|---|---|---|---|
| Positive Deviation | Negative Deviation | ||
| 1. | Obey Raoult's law | Disobey Raoult's law | Disobey Raoult's law |
| 2. | |||
| 3. | |||
| 4. |
Interaction e.g., Chlorobenzene Bromobenzene |
Interaction and e.g., |
Interaction and e.g., |
| 5. |  |
 |
 |
6. Colligative Properties:
A colligative property of a solution is one that depends on the number of particles of solute in solution.
(i) Relative lowering of vapour pressure, .
(ii) Osmotic pressure, .
(iii) Elevation of boiling point,
(iv) Depression in freezing point, .
7. Relative Lowering of :
The relative lowering in of an ideal solution is equal to the mole fraction of solute at that temperature.
for dilute solutions.
Determination of molecular masses by relative lowering in vapour pressure.
Wt. of solute
Mol. wt. of solute
Wt. of solvent
Mol. wt. of solvent
8. Osmotic Pressure:
(i)
Where, density of soln., equilibrium height.
(ii) Van’t Hoff Formula (For calculation of )
(just like ideal gas equation)
total conc. of all types of particles.
Note: If of conc. of conc. are mixed
9. Type of Solutions:
(i) Isotonic solution - Two solutions having same osmotic pressure.
(at same temp.)
(ii) Hypertonic - If solution is hypertonic solution w.r.t. solution.
(iii) Hypotonic – If solution is hypotonic w.r.t. solution.
10. Elevation of Boiling Point:
Where, : Molal elevation constant or ebullioscopic constant, it is the increase in boiling point when the molality of the solution is unity.
, when ,
11. Depression in Freezing Point:
.
Whdere, Molal depression constant. or cryoscopic constant, it is the decrease in freezing point when the molality of solution is unity.
when, ,
Note: and are intensive properties of solvent and do not depend upon the quantity and nature of solute.
12. Abnormal Molecular mass and Van't Hoff Factor :
Since Colligative property
if , no molecular association or dissociation takes place.
if , molecular association takes place.
if , molecular dissociation takes place.
For substances undergoing association or dissociation in the solution.
13. Relation between degree of association or dissociation & Van't Hoff's Factor :
For association or
where Number of particles that associate.
For dissociation or
where Number of particles obtained on dissociation.
