EASY
Earn 100
A balloon filled with an ideal gas shrink when cooled. When shrinking completes, which of the following is same as it was originally?
(a)The velocity of gas particles.
(b)The density of gas.
(c)The pressure in the balloon.
(d)The kinetic energy of gas particle.
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
EASY
Modern vacuum pumps can evacuate a vessel down to a pressure of atm. at room temperature (300 K). Taking R = 8.3 JK-1 mole-1, 1 atm = 105 Pa and , the mean distance between molecules of gas in an evacuated vessel will be of the order of :
EASY
In a closed vessel, an ideal gas at is heated from , the final pressure of the gas will approximately be
MEDIUM
The density of acetic acid vapour at and is . The number of acetic acid molecules in the cluster that is formed in the gas phase is closest to
EASY
The value of 1 mole of any pure ideal gas at standard temperature and pressure is always equal to
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
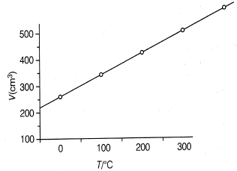
The volume of the gas at is larger than that at by a factor of
EASY
Equal masses of and methane have been taken in a container of volume at temperature in identical conditions. The ratio of the volumes of gases methane would be
EASY
At , the density of a certain gaseous molecule at bar is double to that of dinitrogen at bar. The molar mass of the gaseous molecule is
HARD
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
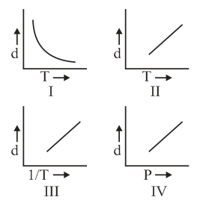
EASY
An ideal gas has pressure , volume and absolute temperature . If is the mass of each molecule and is the Boltzmann constant then density of the gas is
EASY
10 moles of a mixture of hydrogen and oxygen gases at a pressure of 1 atm at a constant volume and temperature, react to form 3.6 g of liquid water. The pressure of the resulting mixture will be closest to:
MEDIUM
What is the density of water vapour at boiling point of water?
MEDIUM
An open vessel at is heated until two fifth of the air (assumed as an ideal gas) in it has escaped from the vessel. Assuming that the volume of the vessel remains constant, the temperature to which the vessel has been heated is:
MEDIUM
A mixture of gases and are taken in a closed vessel containing charcoal. The graph that represents the correct behaviour of pressure with time is:
HARD
Two closed bulbs of equal volume containing an ideal gas initially at pressure and temperature are connected through a narrow tube of negligible volume, as shown in the figure below. The temperature of one of the bulbs is then raised to The final pressure is:
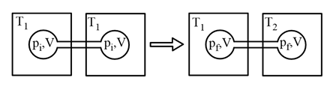
EASY
Which of the following equations does NOT represent Charles’s law for a given mass of gas at constant pressure?
HARD
Aluminium reacts with sulfuric acid to form aluminium sulfate and hydrogen. What is the volume of hydrogen gas in liters produced at and atm pressure, when of aluminium and of sulfuric acid are combined for the reaction? (Use molar mass of aluminium as , )
EASY
A spherical balloon of radius containing helium gas has a pressure of bar. At the same temperature, the pressure, of a spherical balloon of radius containing the same amount of gas will be bar.
MEDIUM
At a constant pressure P, the plot of volume (V) as a function of temperature (T) for 2 moles of an ideal gas gives a straight line with a slope . The value of P (in atm) is closest to
[Gas constant, ]
MEDIUM
Assuming ideal gas behavior, the ratio of density of ammonia to that of hydrogen chloride at same temperature and pressure is: (Atomic weight of Cl is 35.5 u)
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

