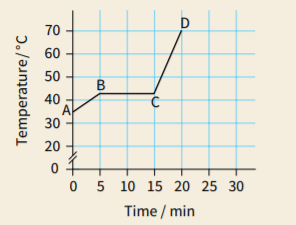
A block of paraffin wax was heated gently, at a steady rate. Heating was continued after the wax had completely melted. The graph shows how the material's temperature varied during the experiment.
For each section of the graph (AB, BC and CD), describe the state of the material.


Important Questions on Thermal Physics
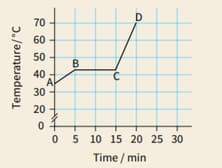
A block of paraffin wax was heated gently, at a steady rate. Heating was continued after the wax had completely melted. The graph shows how the material's temperature varied during the experiment.
For each section, explain whether the material's internal energy is increasing, decreasing or remaining constant.
A block of paraffin wax was heated gently, at a steady rate. Heating was continued after the wax had completely melted. The graph shows how the material's temperature varied during the experiment.
Consider the two sloping sections of the graph.State whether the material's specific heat capacity is greater when it is a solid or when it is a liquid. Justify your answer.
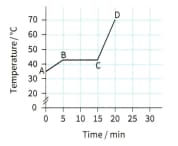
Temperature variation of a sample of wax, heated at a constant rate.
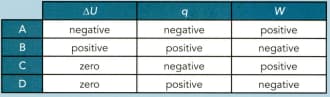
The first law of thermodynamics can be represented by the expression:
An ideal gas is compressed at constant temperature.
Which row shows whether and are negative, positive or zero during the change?
What is the internal energy of an object?