HARD
JEE Main/Advance
IMPORTANT
Earn 100
A buffer solution is prepared by mixing moles of and moles of such that into water to make buffer solution. If the instantaneous (differential) buffer capacity of this buffer solution is plotted against moles of salt then the plot obtained will be (to the scale) approximately:
(a)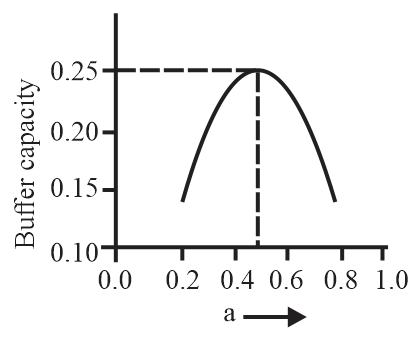

(b)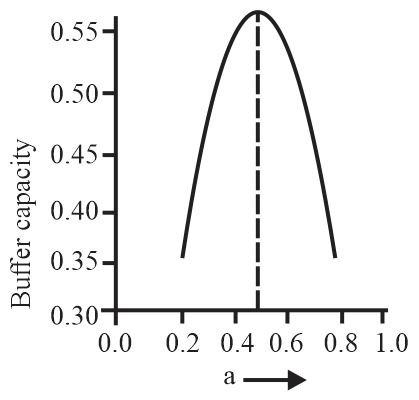

(c)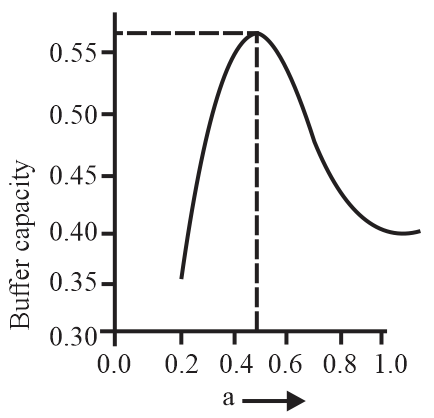

(d)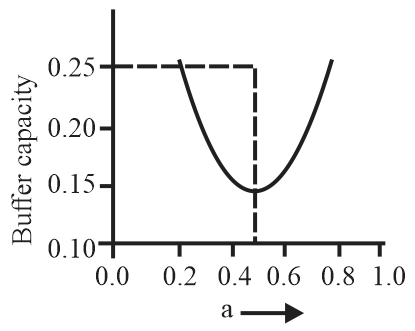

50% studentsanswered this correctly

Important Questions on Equilibrium
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
At constant temperature, the equilibrium constant () for the decomposition reaction: is expressed by , where is the pressure and is the extent of decomposition. Which of the following statements is true?
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
