EASY
VITEEE
IMPORTANT
Earn 100
A compound is soluble in concentrated . It does not decolourise bromine in carbon tetrachloride but is oxidised by chromic anhydride in aqueous sulphuric acid within two seconds, turning orange solution to blue, green and then, opaque. The original compound is
(a)a primary alcohol.
(b)a tertiary alcohol.
(c)an alkane.
(d)an ether.
50% studentsanswered this correctly

Important Questions on Alcohols, Phenols and Ethers
HARD
VITEEE
IMPORTANT
What is in the following sequence of reactions?
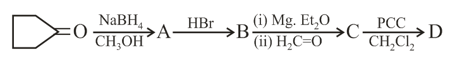
EASY
VITEEE
IMPORTANT
Ethylene glycol, on oxidation with per-iodic acid, gives
MEDIUM
VITEEE
IMPORTANT
In the reaction,
the compound is:
HARD
VITEEE
IMPORTANT
The cresol reacts with chloroform in alkaline medium to give the compound , which adds hydrogen cyanide to form the compound . The latter, on acidic hydrolysis gives chiral carboxylic acid. The structure of the carboxylic acid is
HARD
VITEEE
IMPORTANT
What is in the following sequence of reactions?
HARD
VITEEE
IMPORTANT
-cresol reacts with chloroform in an alkaline medium to give compound , which adds hydrogen cyanide to form compound . The latter on acidic hydrolysis gives chiral carboxylic acid. What is the structure of carboxylic acid?
EASY
VITEEE
IMPORTANT
When acetylene is passed into methanol at in the presence of a small amount of potassium methoxide under pressure, the following is formed:
EASY
VITEEE
IMPORTANT
In Williamson synthesis, if a tertiary alkyl halide is used, then
