HARD
Earn 100
A compound strontium carbide when treated with water it produces an organic compound A which can also be prepared by reaction of 2,3-dichloro ethane with potassium tertiary butoxide. Which of the following is correct for A ?
I. A is an unsaturated compound
Ii. A is acidic in nature
III. A is a saturated compound
IV. A undergoes cis hydroxylation on reaction with OsO4
(a)I and II
(b)II and III
(c)I, II and IV
(d)All of these
42.86% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
trans
EASY
What is the final major product of the following reaction?
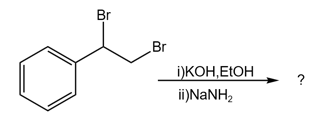
MEDIUM
MEDIUM
In the reaction
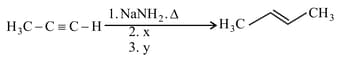
and , respectively, are
MEDIUM
In the following reaction sequence
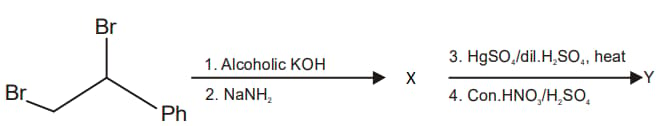
X and Y are, respectively
MEDIUM
MEDIUM
HARD
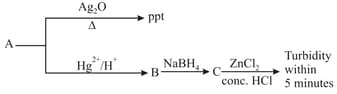
‘A’ is:
EASY
MEDIUM
The major product of the following reaction is
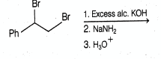
EASY
Which among the following depicts the correct order of acidity?
MEDIUM
EASY
EASY
MEDIUM
The major product of the following reaction is
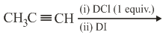
MEDIUM
MEDIUM
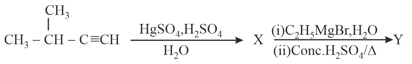
MEDIUM
For the given reaction:

What is ?
MEDIUM
EASY

