A flask of volume is filled with helium gas at r.t.p. Calculate the mass of helium present in the flask. ( value: ).

Important Questions on Atoms, Molecules and Stoichiometry
of a gaseous hydride of phosphorus, reacts with exactly of chlorine, , to form liquid phosphorous trichloride and of hydrogen chloride gas, . How many moles of chlorine react with mole of the gaseous hydride?
of a gaseous hydride of phosphorus, reacts with exactly of chlorine, , to form liquid phosphorous trichloride and of hydrogen chloride gas, . Deduce the formula of the phosphorus hydride.
Boron is present in compounds called borates. The accurate relative atomic mass of iron, , is . Explain why the accurate relative atomic mass is not a whole number.
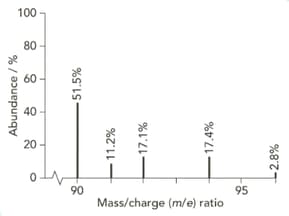
The mass spectrum of zirconium is shown below.
Give the isotopic symbol for the most abundant isotope of zirconium.
A sample of of tin(IV) oxide is mixed with of carbon and heated. A reaction occurs.
Show by calculation that the reagent in excess is tin(IV) oxide. ( values: ).