MEDIUM
JEE Main
IMPORTANT
Earn 100
A gas at absolute temperature has pressure . Boltzmann constant . The number of molecules per is of the order of
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
EASY
JEE Main
IMPORTANT
The number of molecules in of water is closed to
EASY
JEE Main
IMPORTANT
The number of molecules per unit volume of a gas is given by
EASY
JEE Main
IMPORTANT
A gas has volume and pressure . The total translational kinetic energy of all the molecules of the gas is
EASY
JEE Main
IMPORTANT
Two identical containers joined by a small pipe initially contain the same gas at pressure and absolute temperature . One container is now maintained at the same temperature while the other is heated to . The common pressure of the gases will be
MEDIUM
JEE Main
IMPORTANT
A cylindrical steel plug is inserted into a circular hole of diameter in a brass plate. When the plug and the plates are at a temperature of the diameter of the plug is smaller than that of the hole. The temperature at which the plug will just fit in it is
EASY
JEE Main
IMPORTANT
A chamber containing a gas was evacuated till the vacuum attained was of . If the temperature of the chamber was , the number of molecules that remains in it per cubic metre is
EASY
JEE Main
IMPORTANT
An ideal gas is initially at temperature and volume . Its volume is increased by due to an increase in temperature , pressure remaining constant. The quantity varies with temperature as
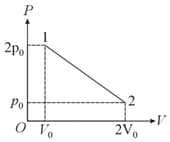
MEDIUM
JEE Main
IMPORTANT
diagram was obtained from state to state when a given mass of a gas is subjected to temperature changes. During this process, the gas is