HARD
Physics
IMPORTANT
Earn 100
A gas consisting of monatomic molecules (degrees of freedom ) was expanded in a polytropic process so that the rate of collisions of the molecules against the vessel's wall did not change. Find the molar heat capacity of the gas in the process, in . (Given: Gas constant )
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
Physics
IMPORTANT
A gas consisting of rigid diatomic molecules was expanded polytropic process so that the rate of collisions of the molecules against the vessel's wall did not change. Find the molar heat capacity of the gas in this process in . (Given: Gas constant )
MEDIUM
Physics
IMPORTANT
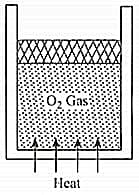
A vertically oriented cylindrical vessel containing oxygen gas and closed by a piston of mass . Piston can slide smoothly in the cylinder. Its cross-sectional area is and atmospheric pressure is . Some heat is supplied to the cylinder, so that the piston is slowly displaced up by . Find the amount of heat supplied to the gas (in Joules).
EASY
Physics
IMPORTANT
Two moles of helium gas are taken over the cycle , as shown in below in diagram.
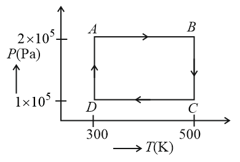
Assuming the gas to be ideal, the work done on the gas in taking it from to is
MEDIUM
Physics
IMPORTANT
Two moles of helium gas is taken over the cycle , as shown in the diagram.
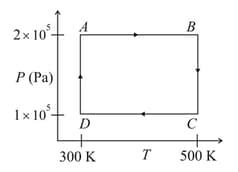
The work done on the gas in taking it from to is
MEDIUM
Physics
IMPORTANT
Two moles of helium gas is taken over the cycle , as shown in the diagram.
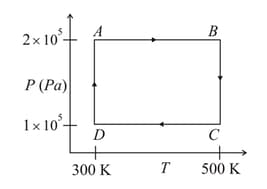
The net work done on the gas in the cycle is
HARD
Physics
IMPORTANT
A diatomic ideal gas is used in a Carnot engine as the working substance. If during the adiabatic expansion part of the cycle, the volume of the gas increases from to , the efficiency of the engine is
HARD
Physics
IMPORTANT
A thermally insulated vessel contains an ideal gas of molecular mass and a ratio of specific heats . It is moving with speed and is suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases by,
MEDIUM
Physics
IMPORTANT
A carnot engine operating between temperatures and has efficiency . When is lowered by , its efficiency increases to . Then and are, respectively
