MEDIUM
BITSAT
IMPORTANT
Earn 100
A graph is plotted between log versus for calculation of activation energy . The correct plot is
(a)
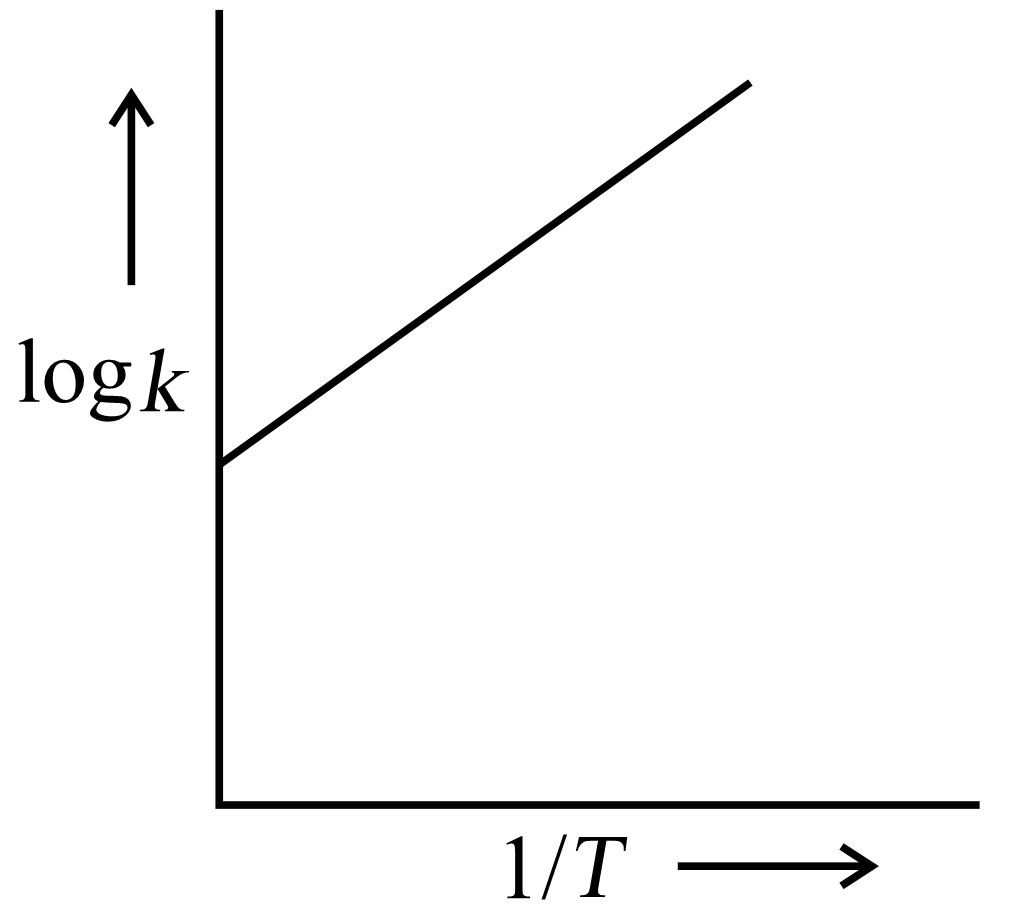
(b)
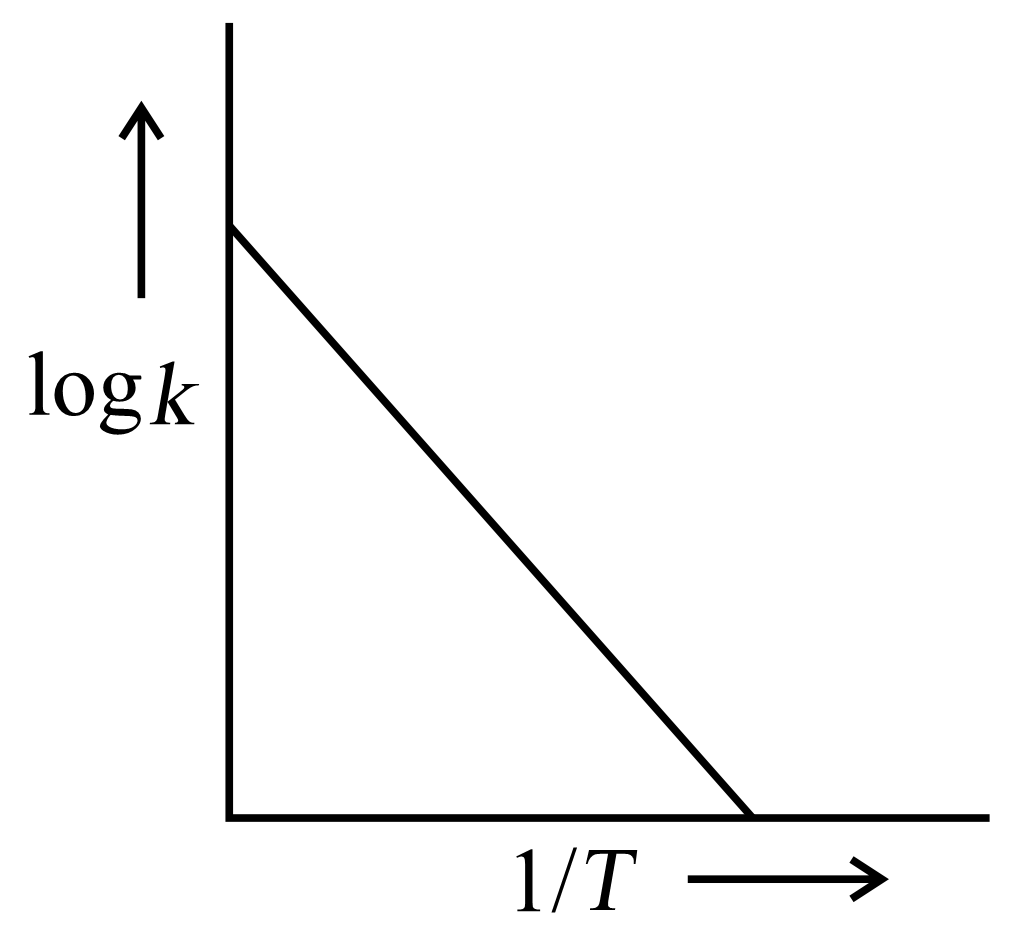
(c)
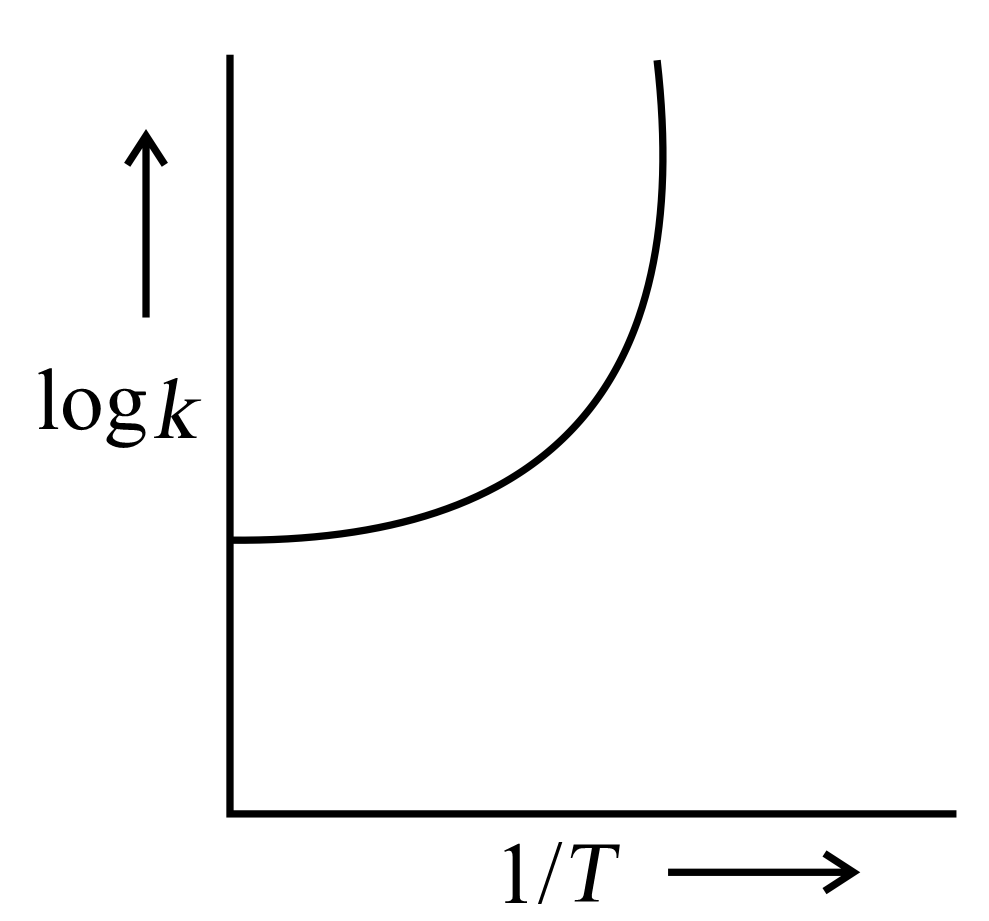
(d)
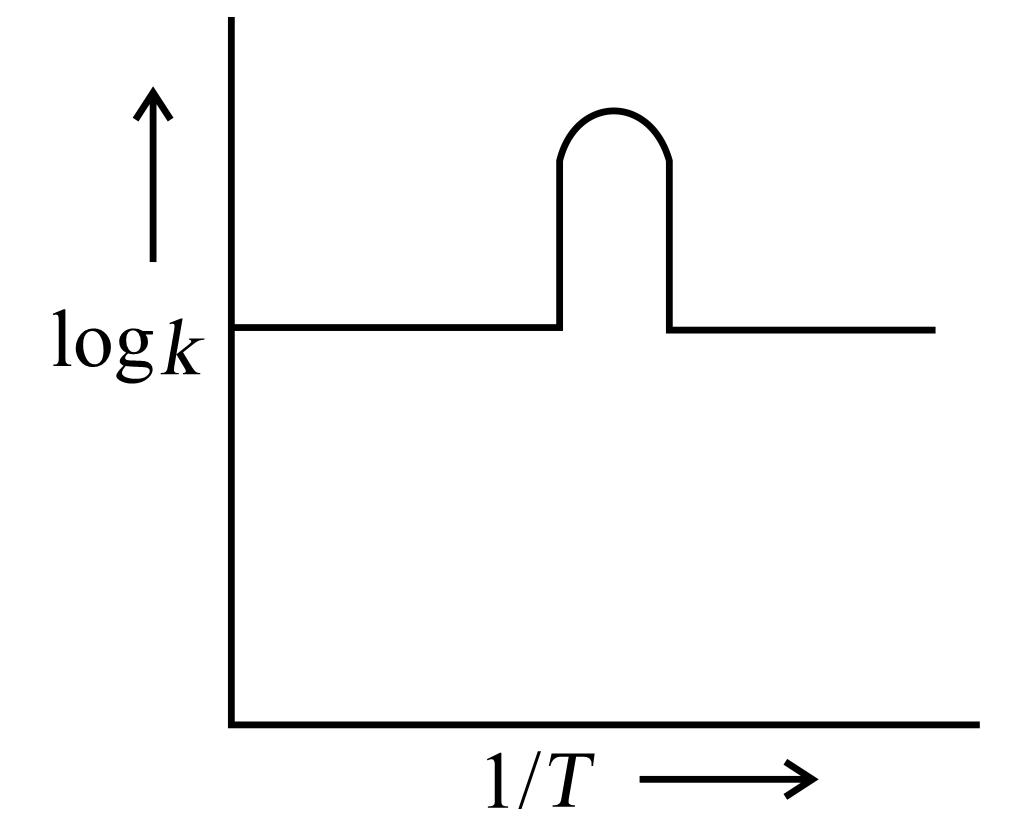
60% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
BITSAT
IMPORTANT
Given the hypothetical reaction mechanism
and the rate as
| Species formed | Rate of its information |
| per mole of | |
| per mole of | |
| per mole of | |
| per mole of |
The rate determining step is
MEDIUM
BITSAT
IMPORTANT
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
HARD
BITSAT
IMPORTANT
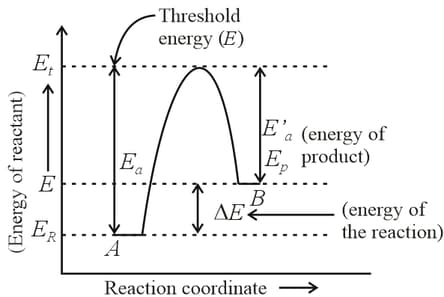
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
MEDIUM
BITSAT
IMPORTANT
Under the same reaction conditions, initial concentration of of a substance becomes half in and through first order and zero order kinetics, respectively. Ratio of the rate of constants for first order and zero order of the reaction is
MEDIUM
BITSAT
IMPORTANT
The rate of a reaction triples when temperature changes from to . The energy of activation for the reaction is
EASY
BITSAT
IMPORTANT
Which of the following expression is correct for the rate of reaction given below ?
MEDIUM
BITSAT
IMPORTANT
For an endothermic reaction, where represent the enthalpy of reaction in , the minimum value for energy of activation (for forward reaction) will be
MEDIUM
BITSAT
IMPORTANT
A graph is plotted between log versus for calculation of activation energy . The correct plot is
