HARD
Earn 100
A layered sequence for an metal is shown below :
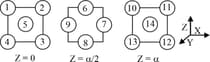
A face diagonal passes through the centre of atom 4 and the centre (s) of which other atom (s)?
(a)1
(b)2, 5
(c)8, 12
(d)9, 10
50% studentsanswered this correctly
Important Questions on The Solid State
HARD
MEDIUM
EASY
HARD
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
HARD
HARD
EASY
MEDIUM
MEDIUM
MEDIUM

