MEDIUM
JEE Main
IMPORTANT
Earn 100
A mixture of gases and are taken in a closed vessel containing charcoal. The graph that represents the correct behaviour of pressure with time is:
(a)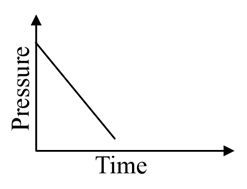

(b)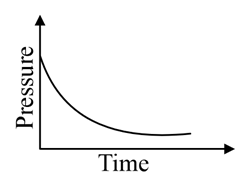

(c)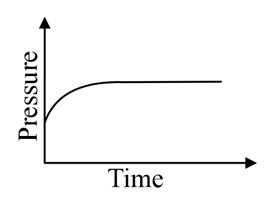

(d)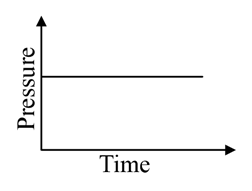

42.86% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
EASY
JEE Main
IMPORTANT
moles of gas and moles of gas exert a pressure of in a container of volume at . Given, is the gas constant in is:
EASY
JEE Main
IMPORTANT
At a given temperature gases and are found to deviate from ideal gas behaviour. Their equation of state is given as at Here, is the van der Waals constant. Which gas will exhibit steepest increase in the plot of (compression factor) vs
MEDIUM
JEE Main
IMPORTANT
Consider the van der Waal's constants, and for the following gases.
| Gas | ||||
|---|---|---|---|---|
Which gas is expected to have the highest critical temperature?
EASY
JEE Main
IMPORTANT
At , the density of a certain gaseous molecule at bar is double to that of dinitrogen at bar. The molar mass of the gaseous molecule is
EASY
JEE Main
IMPORTANT
At very high pressures, the compressibility factor of one mole of a gas is given by:
EASY
JEE Main
IMPORTANT
Which intermolecular force is most responsible in allowing xenon gas to liquefy?
MEDIUM
JEE Main
IMPORTANT
The temperature at which oxygen molecules have the same root mean square speed as helium atoms have at 300 K is : (Atomic masses : He = 4 u, O = 16 u)
HARD
JEE Main
IMPORTANT
Van der Waal's equation for a gas is stated as,
.
This equation reduces to the perfect gas equation, When,
