EASY
JEE Main
IMPORTANT
Earn 100
A monoatomic ideal gas, initially at temperature , is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature by releasing the piston suddenly. If and are lengths of the gas column before and after expansion respectively, then is given by
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
EASY
JEE Main
IMPORTANT
Starting with the same initial conditions, an ideal gas expands from volume to in three different ways. The work done by the gas is if the process is isothermal, if isobaric and if adiabatic, then
EASY
JEE Main
IMPORTANT
and are two adiabatic curves for two different gases. Then and corresponds to
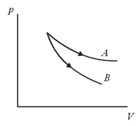
EASY
JEE Main
IMPORTANT
Choose the incorrect statement related to an isobaric process.
EASY
JEE Main
IMPORTANT
The amount of heat required to raise the temperature of 1 mole of a monoatomic gas from to at constant volume is . Then, the amount of heat required to raise the temperature of mole of a diatomic gas from to at constant pressure is
EASY
JEE Main
IMPORTANT
Specific heat of gas undergoing adiabatic changes is
EASY
JEE Main
IMPORTANT
During an isothermal expansion of an ideal gas
EASY
JEE Main
IMPORTANT
Two identical samples of gas are allowed to expand
(i) isothermally
(ii) adiabatically.
Work done is
EASY
JEE Main
IMPORTANT
A given system undergoes a change in which the work done by the system equals the decrease in its internal energy. The system must have undergone an
