MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A monoatomic ideal gas undergoes a thermodynamic process according to the relation constant. If the molar heat capacity of this gas at constant volume is then its molar heat capacity for the given process will be
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
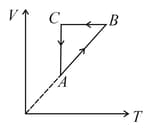
A thermodynamic cycle on an ideal gas is shown in the $V-T$ graph. Which of the following $P-T$ graph represents the same cycle?
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
