HARD
Earn 100
A nitrile on acid hydrolysis gives compound 'A', which reacts with thionyl chloride to give compound 'B'. Benzene reacts with compound 'B' in presence of anhydrous to give compound 'C'. Identify the compounds 'A', 'B' and 'C' and write the equations.
Important Questions on Aldehydes, Ketones and Carboxylic Acids
MEDIUM
The major product of the following reaction is:
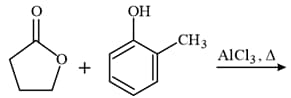
MEDIUM
In the following sequence of reactions:
The Product is:
MEDIUM
Identify and in the following reaction
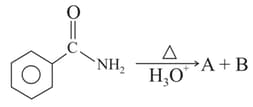
EASY
Which among the following compounds is obtained when ethane nitrile is acid hydrolysed?
EASY
Which one of the following compounds reacts with phenyl magnesium bromide to form a salt of benzoic acid?
MEDIUM
Hydrolysis of cyanohydrin derivative produces
EASY
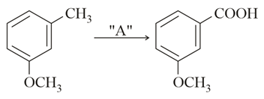
In the above reaction, the reagent "" is :
MEDIUM
What is the product of following reaction?
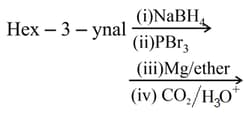
HARD
An organic compound [A], molecular formula was hydrolyzed with dilute sulphuric acid to given a carboxylic acid [B] and an alcohol [C]. Oxidation of [C] with produced [B]. Which of the following structures are not possible for
MEDIUM
The compound in the following reaction scheme:
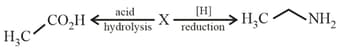 is:
is:
MEDIUM
The major product formed when 1, 1, 1- trichloropropane is treated with aqueous potassium hydroxide is :
MEDIUM
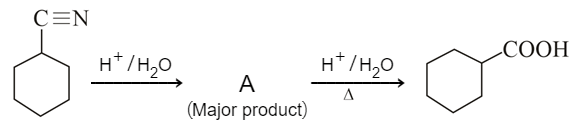
Consider the above chemical reaction and identify product
MEDIUM
Which of the following reactions does NOT yield an amine
MEDIUM
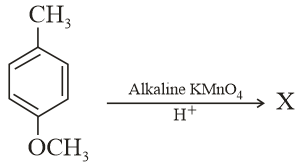
Considering the above chemical reaction, identify the product
MEDIUM
Which of the following compound upon oxidation gives isophthalic acid?
HARD
How will you bring about the following conversion:
Benzaldehyde to benzophenone?
HARD
An organic compound (A) (molecular formula ) was hydrolysed with dil. to give a carboxylic acid (B) and an alcohol (C). ‘ ’ gives white turbidity immediately when treated with anhydrous and conc. . The organic compound (A) is:
MEDIUM
The compound formed as a result of oxidation of ethyl benzene by is
MEDIUM
In the reaction
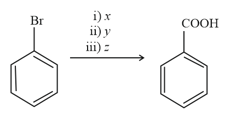
and are

