A radical with one atom of carbon and three atoms of oxygen is ______.
Important Questions on Theoretical Principles of Experimental Chemistry
Given below are two statements:
Statement-I : Methyl orange is a weak acid.
Statement-II : The benzenoid form of methyl orange is more intense/deeply coloured than the quinonoid form.
In the light of the above statement, choose the most appropriate answer from the options given below:
A metal chloride shows the following reactions:
(i) When is passes in an acidified aqueous solution of a black ppt is a obtained.
(ii) The precipitate obtained in step (i) is not soluble in yellow ammonium sulphide.
(iii) When a solution of stannous chloride is added to an aqueous solution of ,a white precipitate is obtained which turns grey on addition of more of stannous chloride.
(iv) When an aqueous solution of is added to an aqueous solution of , a red precipitate is obtained which dissolves on addition of excess of .
Identify and write down the equations for the reaction at steps (i), (iii) & (iv).
Match the following:-
| Column I | Column II | |
| (Elements) | (Symbol) | |
| (i) | Acidic Radicals | |
| (ii) | Basic Radicals | |
| (iii) | Monovalent Radicals | |
| (iv) | Variable Valency | |
| (v) | Tetravalent elements |
An aqueous solution of an unknown compound gives the following reactions.
(i) It gives brown precipitate with alkaline solution
(ii) It forms & evolved when reacts with gas.
(iii) It liberates from an acidified solution.
(iv) It gives orange yellow colour with acidified titanic sulphate solution.
Identify and give the chemical equations for the reactions (i), (ii) & (iii).
Identify the odd one out and justify.
Chloride, nitrate, hydride, ammonium.
Select the incorrect statement with regards to Indigo .
Give examples of composite radicals.
Give examples of metals with variable valency.
(i) Cu+ undergoes disproportionation to Cu and Cu2+ in aqueous solution
(ii) Hg2Cl2 does not impart chromyl chloride test
(iii) Sulphide ions react with sodium nitroprusside to form a purple coloured complex. In this reaction, oxidation state of iron changes.
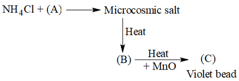
In the above given reaction reactant (A) and product (B) and (C) are :

