HARD
11th CBSE
IMPORTANT
Earn 100
A reversible cyclic process for an ideal gas is shown below. Here, are pressure, volume and temperature respectively. The thermodynamic parameters of are heat, work, enthalpy and internal energy respectively
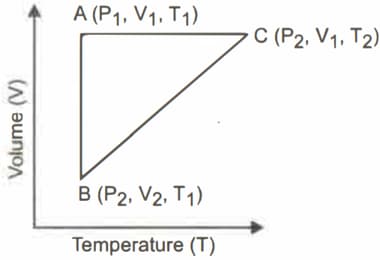
The correct option(s) is (are)

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
11th CBSE
IMPORTANT
For a reaction , the plots of with time at temperatures and are given below:
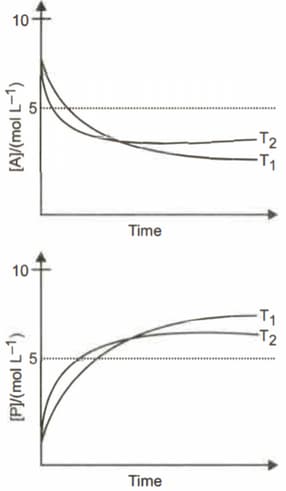
If , the correct statement(s) is (are) (Assume and are independent of temperature and ratio of at to at is greater than. Here, are enthalpy, entropy, Gibbs energy and equilibrium constant respectively)
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
The number of properties which are state functions among the following is
Pressure, Volume, Temperature, Heat, Work, Entropy, Enthalpy, Free energy, Internal energy.
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
