MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A small particle of mass moves in such a way that the potential energy , where is a constant and is the distance of the particle from the origin (Nucleus). Assuming Bohr model of quantization of angular momentum and circular orbits, show that the radius of the allowed orbit is proportional to .

Important Questions on Atomic Physics
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Suppose in certain conditions only those transitions are allowed to hydrogen atoms in which the principal quantum number change by .
(i) Find the smallest wavelength emitted by hydrogen
(ii) List the wavelengths emitted by hydrogen in the visible range ( to ) ()
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
An electron in the ground state of hydrogen atoms is revolving in anti clock wise direction in a circular orbit of radius .
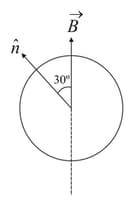
(i) Obtain an expression for the orbital magnetic dipole moment of the electron.
(ii) The atom is placed in a uniform magnetic induction, such that the plane normal to the electron orbit makes an angle of with the magnetic induction. Find the torque experienced by the orbiting electron.
EASY
JEE Main/Advance
IMPORTANT
