EASY
AS and A Level
IMPORTANT
Earn 100
A student calculated the standard enthalpy change of combustion of ethanol by calorimetry as . The data book value is . Explain why there is a difference between these values.

Important Questions on Enthalpy Changes
EASY
AS and A Level
IMPORTANT
Draw an enthalpy cycle to calculate for the reaction, .
EASY
AS and A Level
IMPORTANT
Calculate using the following information,
EASY
AS and A Level
IMPORTANT
Draw an enthalpy cycle to calculate the enthalpy change of formation of ethanol, , using enthalpy changes of combustion.
EASY
AS and A Level
IMPORTANT
Calculate a value for using the following data:
EASY
AS and A Level
IMPORTANT
Look at this equation.
.
Which one of the following gives the correct value for the enthalpy change of this reaction?
EASY
AS and A Level
IMPORTANT
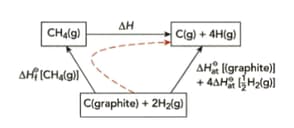
Use the information in Figure and the information below to demonstrate that the average bond energy of the bond is .
EASY
AS and A Level
IMPORTANT
Look at this equation.
.
Which one of the following statements is completely correct?
EASY
AS and A Level
IMPORTANT
Define standard enthalpy change of combustion.
