A student investigated the effect of several different catalysts on the rate of decomposition of hydrogen peroxide to water and oxygen. The speed of the reaction was judged by how 'fizzy' or frothy the contents of the tube became when the catalyst was added (oxygen is a product of the reaction and forms bubbles). The student used iron filings and manganese dioxide as inorganic catalysts. They also used a commercial preparation of the enzyme catalase and pieces of liver and pieces of potato tuber, both of which contain catalase. Catalase catalyses the decomposition of hydrogen peroxide.
Results showed: • catalase, liver and potato were much more efficient than the inorganic catalysts • pure catalase was more efficient than the liver and potato • liver was more efficient than potato • ground-up liver was more efficient than pieces of liver. Try to explain the student's results.

Important Questions on Enzymes
Why is it better to calculate the initial rate of reaction from a curve such as the one in. In given figure how much oxygen is given off in 30 seconds?
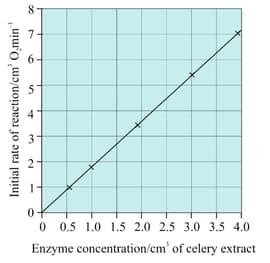
Sketch the shape that would be in the given graph, if excess hydrogen peroxide were not available. (Celery extract contains catalase enzyme).
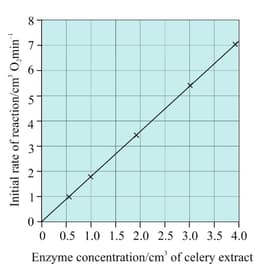
Outline an investigation you could carry out to compare the temperature at which the enzyme lactase is completely denatured within ten minutes
When free in solution
