A student takes and pieces separately in four test tubes labelled as I, II, III, and IV respectively. He adds mL of freshly prepared ferrous sulphate solution to each test tube and observes the colour of the metal residue in each case.
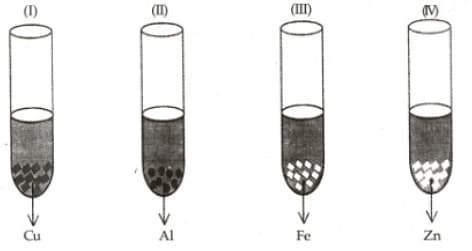
He would observe a black residue in the test tubes:


Important Questions on Multiple Choice Questions(MCQ's)
Four students (A), (B), (C) and (D) observed the colour and solubility of iron, sulphur, and iron sulphite in carbon disulphide. The tick mark represents 'soluble', and the cross mark represents 'insoluble' in carbon disulphide. Their observations are tabulated below:
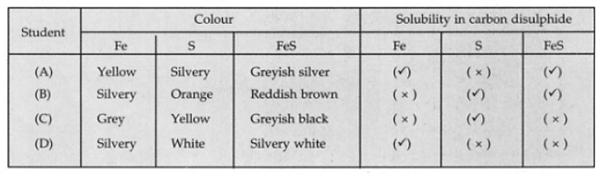
The student, who correctly reported the observations, is a student:
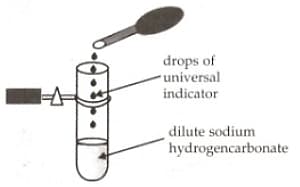
A student adds a few drops of universal indicator solution to a dilute solution of sodium hydrogen carbonate taken in the test-tube. Which of the following colour would be observed?
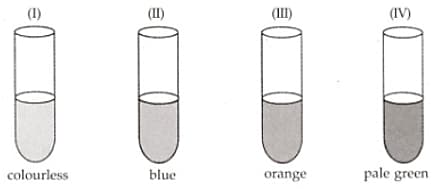
A student took four test-tubes containing solutions of different colours marked I, II, III and IV as shown here. The test-tube containing copper sulphate solution and ferrous sulphate solution could be the tubes:
Four students added a small amount of ethanoic acid to sodium hydrogen carbonate. The gas evolved was tested for its behaviour with burning splinter and lime water. They reported their observations as given:
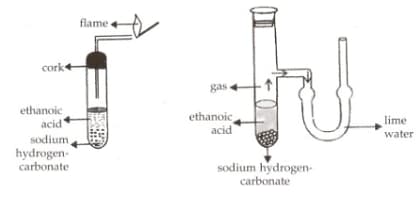

The correct observations have been reported by a student:
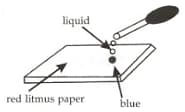
A student placed a few drops of a liquid over a portion of the red litmus paper as shown here. He observed that the red litmus paper turned blue. The liquid could be: