HARD
JEE Main
IMPORTANT
Earn 100
A substance , which sublimes on heating, forms nitrate when treated with and a small quantity of . Suggest from this information a suitable test for .
(a)Nessler's test
(b)Decolorization with acidified
(c)Brown ring test
(d)Lime water test
50% studentsanswered this correctly

Important Questions on Qualitative Inorganic Salt Analysis
MEDIUM
JEE Main
IMPORTANT
Turnbull's blue and Prussian's blue respectively are
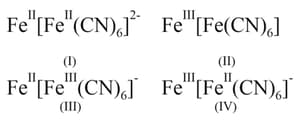
MEDIUM
JEE Main
IMPORTANT
When gas is passed through aqueous solution containing we do not get a precipitate of
MEDIUM
JEE Main
IMPORTANT
Three samples of the same salts are taken separately. Excess of gives white precipitate with the first sample. The second sample gives white precipitate with . The third sample gives black precipitate when gas is passed through the solution. The possible salt is
MEDIUM
JEE Main
IMPORTANT
In the borax bead test of , the blue colour of bead is due to the formation of:
MEDIUM
JEE Main
IMPORTANT
Aq. Solution of reacts with to form yellow precipitate. The cation(s) present in is/are:
MEDIUM
JEE Main
IMPORTANT
Potassium chromate solution is added to aqueous solutions of metal nitrate. The yellow precipitate thus obtained is insoluble in acetic acid. These are subjected to flame test, flame colour of individual precipitate is/are
HARD
JEE Main
IMPORTANT
Which of the following aqueous solution of cation(s) give(s) white precipitate with and solution and formed precipitate is/are further completely dissolved in one of the excess reagent?
MEDIUM
JEE Main
IMPORTANT
Saturated solution of is heated at in a closed container. The product obtained is treated with solution. What is/are the most suitable observation(s)?
