MEDIUM
JEE Advanced
IMPORTANT
Earn 100
A vessel contains a mixture of of nitrogen and of carbon dioxide at temperature . If the pressure of the mixture is , calculate its density

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
MEDIUM
JEE Advanced
IMPORTANT
An electric bulb of the volume was sealed off during manufacture at a pressure of of mercury at . Compute the number of air molecules contained in the bulb. Given that and .
MEDIUM
JEE Advanced
IMPORTANT
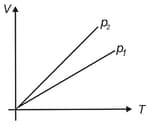
State whether or for given mass of a gas?
EASY
JEE Advanced
IMPORTANT
For a given mass of gas what is the shape of versus graph at constant temperature?
EASY
JEE Advanced
IMPORTANT
For a given mass of a gas, what is the shape of versus graph in the isothermal process?
MEDIUM
JEE Advanced
IMPORTANT
A gas mixture consists of moles of oxygen and moles of argon at temperature . Neglecting all vibrational modes, the total internal energy of the system is
EASY
JEE Advanced
IMPORTANT
The average translational kinetic energy of (molar mass ) molecules at a particular temperature is . The translational kinetic energy of (molar mass ) molecules in at the same temperature is
EASY
JEE Advanced
IMPORTANT
At a given temperature, the rotational kinetic energy of diatomic gas is . Find its translational and total kinetic energy.
EASY
JEE Advanced
IMPORTANT
Calculate the root mean square velocity of hydrogen molecules at .