MEDIUM
Earn 100
A yellow precipitate of lead chromate formed by the reaction between acetic acid and lead acetate confirms the presence of ions in the salt.
50% studentsanswered this correctly
Important Questions on Inorganic Qualitative Analysis
EASY
MEDIUM
EASY
EASY
HARD
EASY
The blue colour produced when a starch solution is added to a solution containing traces of is due to
(I) formation of
(II) formation of an inclusion complex
(III) Oxidation of starch
(IV) Oxidation of
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
side products
side products
side products
The sum of the total number of atoms in one molecule each of and is ________
MEDIUM
MEDIUM
Match List I with List II.
| List-I (Anion) |
List-II (gas evolved on reaction with dil. ) |
||
| (A) | (I) |
Colourless gas which turns lead acetate paper black. |
|
| (B) | (II) |
Colourless gas which turns acidified potassium dichromate solution green. |
|
| (C) | (III) | Brown fumes which turns acidified KI solution containing starch blue. | |
| (D) | (IV) | Colourless gas evolved with brisk effervescence, which turns lime water milky. |
Choose the correct answer from the options given below
MEDIUM
MEDIUM
MEDIUM
HARD
Distinguish between the following pair of compounds using a reagent as a chemical test:
- Magnesium chloride and magnesium nitrate solution.
HARD
HARD
brown fumes. Which is the correct statement regarding the above observation.
MEDIUM
Some reactions are given for salt(X).
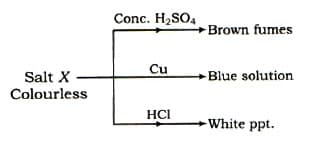
Which of the following salt can be satisfied all the conditions?

