HARD
Earn 100
has type structure. If edge length of crystal lattice is . The radius of (approx)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solid State
MEDIUM
MEDIUM
What is the number of atoms in end centered unit cell?
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
(a) Aluminium crystallizes in a cubic close-packed structure. Its metallic radius is . What is the length of the side of the unit cell?
(b) Why is potassium chloride sometimes violet instead of pure white?
MEDIUM
MEDIUM
HARD
HARD
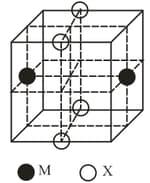
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).