EASY
NEET
IMPORTANT
Earn 100
According to Einstein's photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is,
(a)
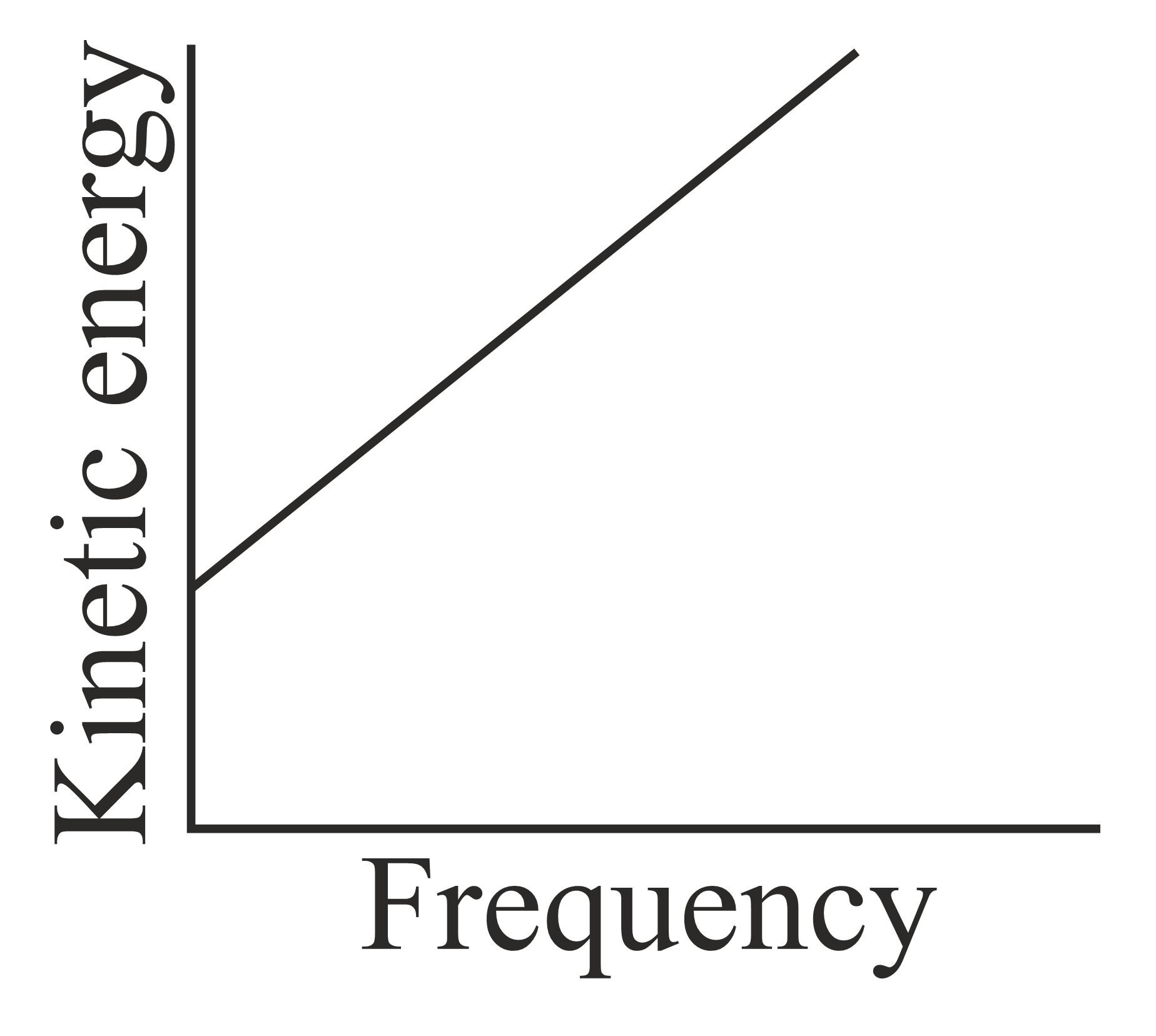
(b)
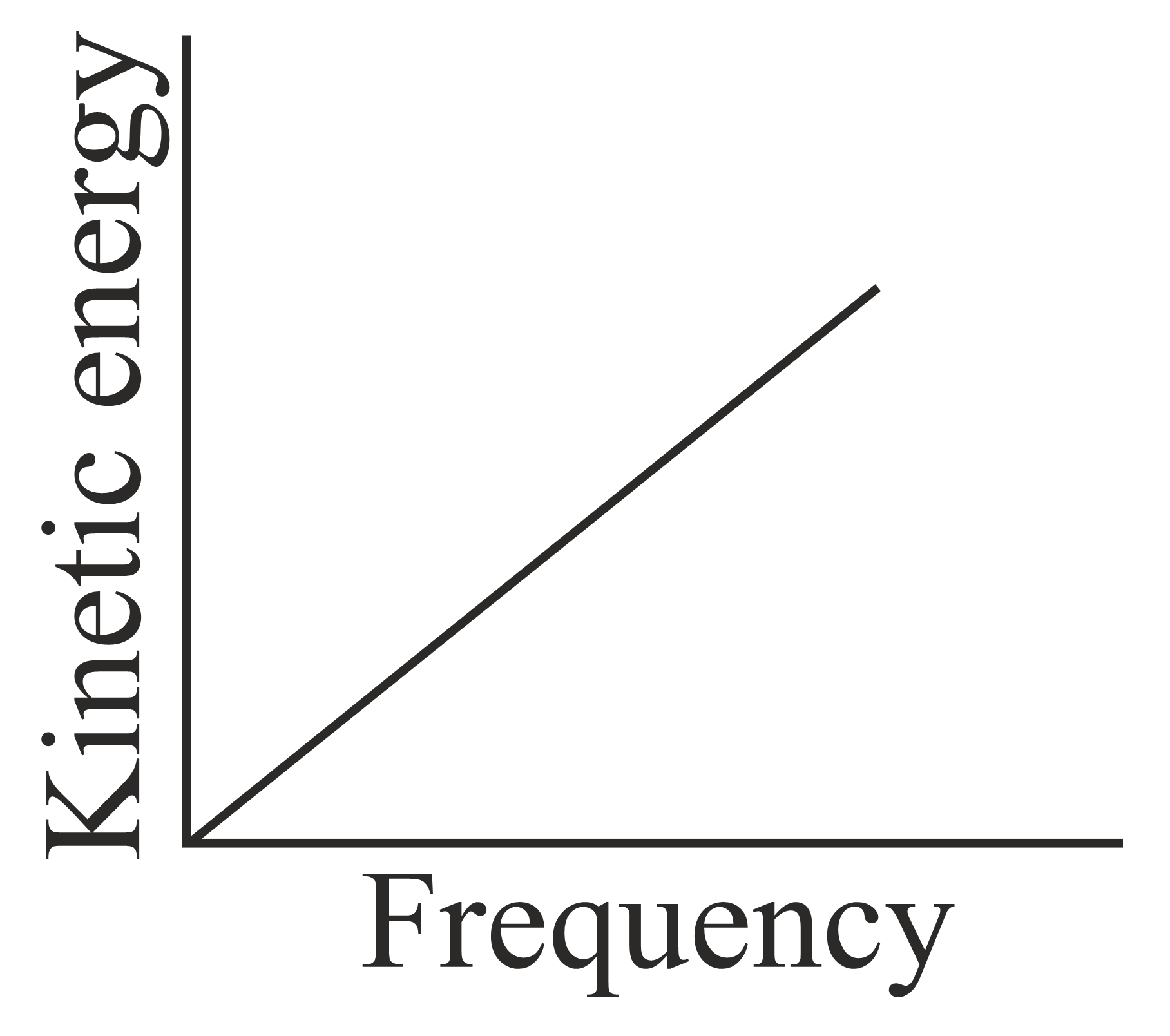
(c)
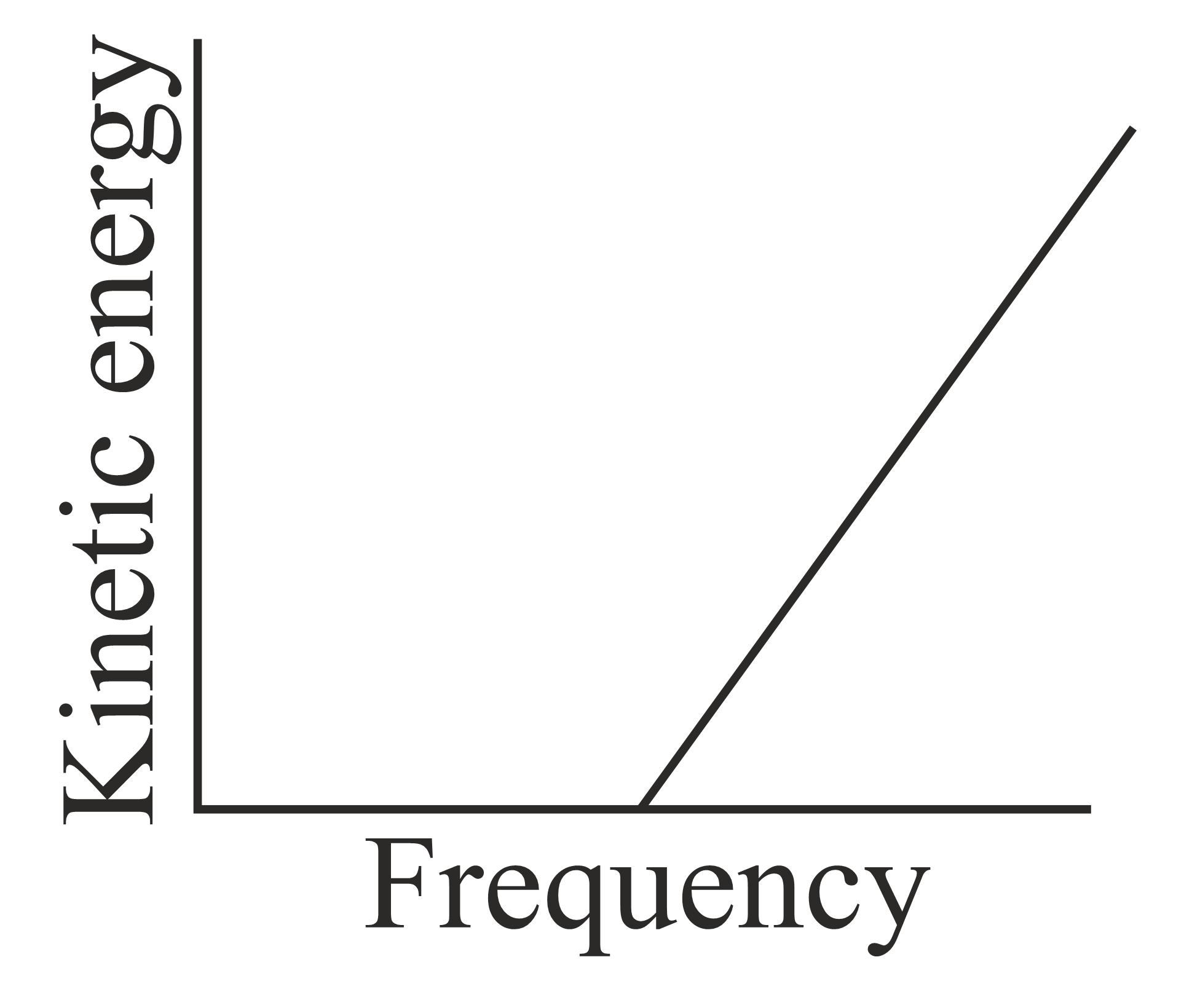
(d)
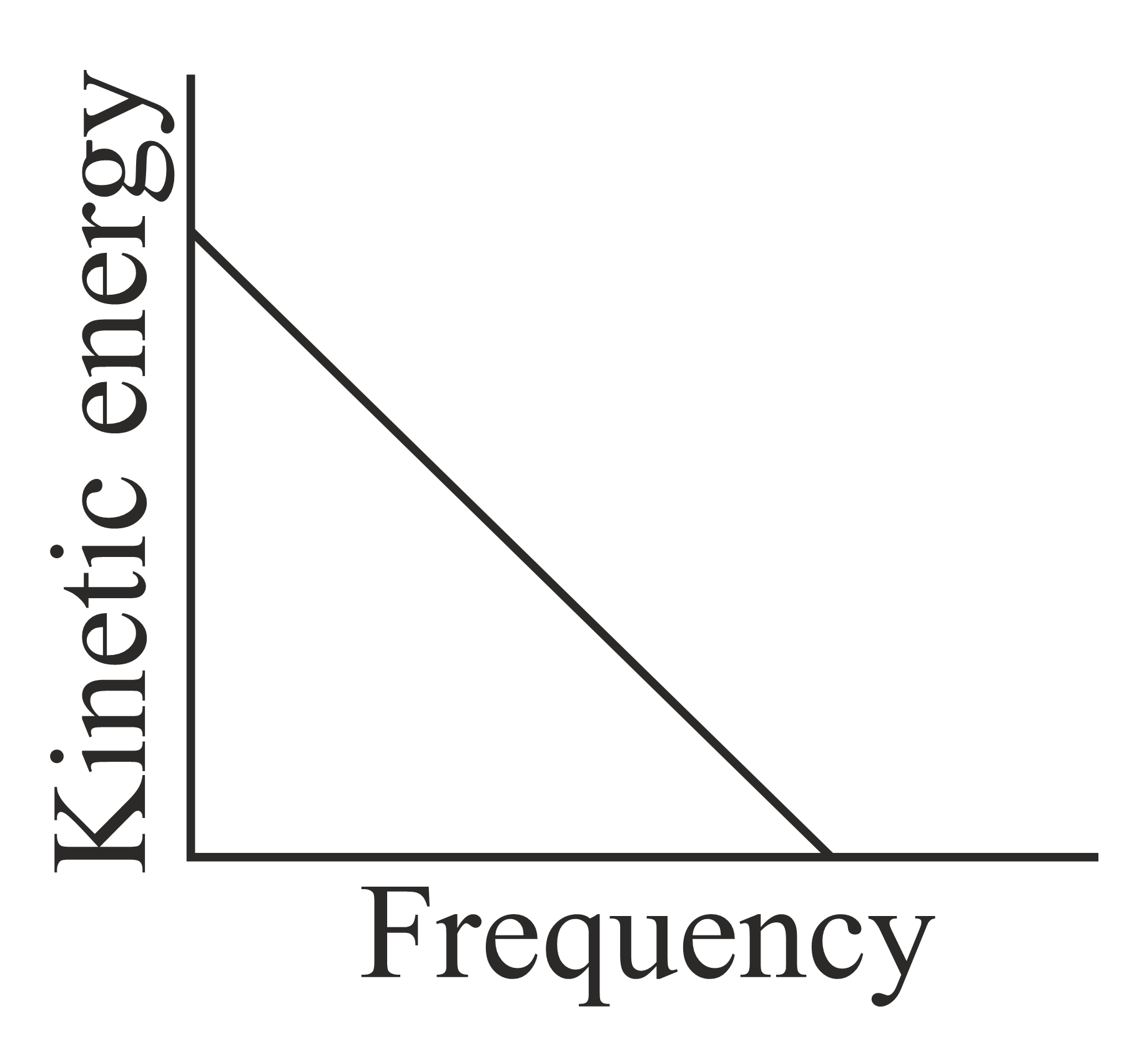
50% studentsanswered this correctly

Important Questions on Dual Nature of Matter and Radiation
EASY
NEET
IMPORTANT
According to Einstein’s photoelectric equation, the plot of the kinetic energy of the emitted photoelectrons from a metal versus the frequency of the incident radiation gives a straight line whose slope,
EASY
NEET
IMPORTANT
A photon of energy is incident on a metal surface whose work function is . The minimum reverse potential to be applied for stopping the emission of electrons is,
EASY
NEET
IMPORTANT
If and are the energy and the momentum of a photon respectively then, on reducing the wavelength of photon,
MEDIUM
NEET
IMPORTANT
If the kinetic energy of a moving particle is , then the de Broglie's wavelength associated with the particle is,
MEDIUM
NEET
IMPORTANT
An electron has an energy of . What will be its wavelength?
EASY
NEET
IMPORTANT
The ratio of wavelength of deuteron and proton accelerated through the same potential difference will be,
EASY
NEET
IMPORTANT
An electron is accelerated from rest between two points with the potentials and respectively. The de-Broglie's wavelength associated with the electron at will be,
EASY
NEET
IMPORTANT
An electron is moving with velocity . The de-Broglie wavelength associated with the electron is (mass of electron, Planck's constant),
