on photographic film is developed with alkaline hydroquinone according to the following reaction
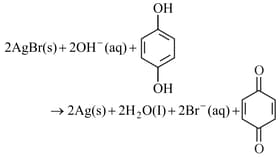
Select the correct statement


Important Points to Remember in Chapter -1 - Redox Reactions from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Concept of oxidation & reduction:
| Oxidation | Reduction | |
|---|---|---|
| (i) | Loss of electron | Gain of electron |
| (ii) | Loss of hydrogen | Gain of hydrogen |
| (iii) | Gain of oxygen | Loss of oxygen |
| (iv) | Increase in oxidation number | Decrease in oxidation number |
2. Oxidation Number:
Oxidation number change is defined as the change (real or imaginary) which an atom appears to have undergone when it is present in redox reaction. There are certain rules laid down in order to determine the oxidation number.
(i) The oxidation number of an atom in free elements is zero.
(ii) The oxidation number of oxygen is , while in peroxides, it is ; in it is .
(iii) The oxidation number of hydrogen is , while in metal hydrides, it is .
(iv) The oxidation number of an ion is equal to the electrical charge present on it.
(v) The oxidation number of group elements is and that of group IIA elements is .
(vi) For complex ions, the algebraic sum of oxidation numbers of all the atoms is equal to the net charge on the ion.
(vii) In case of neutral molecules, the algebraic sum of the oxidation number of all the atoms present in the molecules is zero.
(viii) Oxidation number of an atom never be greater than its valence electron, e.g., valence electron for is .
(ix) An increase in oxidation number of an element in a reaction is known as oxidation while a decrease in oxidation number of an element in a reaction is known as reduction.
(x) Besides positive and negative values, fractional values of oxidation number (as average) are also possible.
(xi) Redox reactions are also called electron - transfer reactions since electrons are transferred from the reductant to the oxidant.
(xii) Oxidation is also called de-electronation while reduction is called electronation.
(xiii) If a compound contains two or more atoms of the same element, all of them may or may not have the same oxidation number e.g.
In , one -atom has oxidation number , while the other has oxidation number .
In i.e., (bleaching powder), oxidation number of one , while oxidation number of the other .
i.e., oxidation number of one while that of each of the other two .
In , oxidation no. of of in .
In (sodium tetrathionate) has the structure of
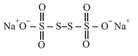
The oxidation number of both atoms in the middle is equal to (pure covalent nature) and other two sulphur atoms at the terminal positions have .
3. Types of Redox Reaction:
(i) Combination Reactions: Chemical reactions in which two or more substances (elements or compounds) combine to form a single substance.
(ii) Decomposition Reactions: Chemical reactions in which a compound breaks up into two or more simple substances.
(iii) Displacement Reactions: Reaction in which one ion (or atom) in a compound is replaced by an ion (or atom) of another element.
(iv) Metal Displacement Reactions: Reactions in which a metal in a compound is displaced by another metal in the uncombined state.
(v) Non-metal Displacement Reactions: Such reactions are mainly hydrogen displacement or oxygen displacement reactions.
(vi) Disproportionation Reactions: Reactions in which an element in one oxidation state is simultaneously oxidized and reduced.
4. Balancing of Redox Reaction:
(i) Steps involved in balancing a redox reaction by oxidation number method:
(a) Write the skeletal redox reaction for all reactants and products of the reaction.
(b) Indicate the oxidation number of all the atoms in each compound above the symbol of an element.
(c) Identify the element/elements which undergo a change in oxidation numbers.
(d) Calculate the increase or decrease in oxidation number per atom.
(e) Equate the increase in oxidation number with a decrease in oxidation number on the reactant side by multiplying formula of oxidizing agent and reducing agents with suitable coefficients.
(f) Balance the equation with respect to all other atoms except hydrogen and oxygen.
(g) Finally, balance hydrogen and oxygen. For balancing oxygen atoms, add water molecules to the side deficient in it. Balancing of hydrogen atoms depend upon the medium.
(h) For reactions taking place in acidic solutions add ions to the side deficient in hydrogen atoms.
(i) For reactions taking place in basic solutions add molecules to the side deficient in hydrogen atoms and simultaneously add equal number of ions on the other side of the equation.
(j) Finally balance the equation by cancelling common species present on both sides of the equation.
(ii) Steps involved in balancing a redox reaction ion-electron method. (Half Reaction method)
(a) Find the elements whose oxidation numbers are changed. Identify the substance that acts as an oxidizing agent and reducing agent.
(b) Separate the complete equation into oxidation half-reaction and reduction half-reaction.
Balance the half-equations by following steps.
(c) Balance all atoms other than and .
(d) Calculate the oxidation number on both sides of the equation. Add electrons to whichever side is necessary to make up the difference.
(e) Balance the half equation so that both sides get the same charge.
(f) Add water molecules to complete the balancing of the equation.
(g) Add the two balanced equations. Multiply one or both half equations by suitable numbers so that on adding two equations the electrons are balanced.
5. Equivalent Weight:
Equivalent weight of substance is defined as the number of parts by weight of given substance which combines or displaces part by weight of hydrogen ( of at ), parts by weight of oxygen ( of at ), parts by weight of chiorine ( of at )
(i) Equivalent weight of element
(ii) Equivalent weight of acids
(iii) Equivalent weight of bases
(iv) Equivalent weight of salts
(v) Equivalent weight of reducing agent
(vi) Equivalent weight of oxidizing agent
6. -factor or Valence Factor:
-factor is very important for both redox and non-redox reactions through which we predict the following two information:
(i) It predicts the molar ratio of the species taking part in reactions i.e., reactants. The reciprocal of -factor's ratio of the reactants is the molar ratio of the reactants.
For example: If (having -factor ) reacts with (having -factor ) then its -factor's ratio is so molar ratio of to is .
It can be represented as
(ii) Equivalent weight or
7. Law of Equivalence:
According to law of equivalence, for each and every reactant and product,
Equivalents of each reactant reacted Equivalents of each product formed.
For example:
Suppose the reaction is taking place as given.
Then, according to law of equivalence,
Equivalents of reacted = Equivalents of reacted Equivalents of produced Equivalents of produced.
8. Application of Redox Reaction:
Redox Titrations:
Potassium permanganate in redox reactions: Potassium permanganate is very strong oxidizing agent and is used in determination of many reducing agents like , oxalate ions etc. It acts as self indicator in redox reactions.
Equation showing as an oxidizing agent in acidic medium is:
(i) Acidified Potassium dichromate in redox reactions:
is used as an oxidizing agent in redox reactions. Titrations involving uses diphenylamine and potassium ferricyanide (external indicator).
Equation showing as an oxidizing agent in acidic medium is:
(ii) Iodine in redox reactions:
acts as mild oxidizing agent in solution according to equation.
(iii) Direct redox reaction:
Redox reactions in which reduction and oxidation occurs in same solution (i.e., same reaction vessel). In these reactions transference of electrons is limited to very small distance.
(iv) Indirect redox reactions:
Redox reactions in which oxidation and reduction reactions take place in different reactions vessels and thus transfer of electrons from one species to another does not take place directly.
9. Electrochemical cell:
Electrochemical cell is a device that converts chemical energy produced in a redox reaction into electrical energy. These cells are also called Galvanic cells or Voltaic cells.
The electrode at which oxidation occurs is called anode and is negatively charged.
The electrode at which reduction takes place is called cathode and is positively charged.
