All matter is made up of atoms and earlier atoms were supposed to be the smallest indivisible particles. But further discoveries showed that this atom can be divided into subatomic particles. So the structure of an atom is quite complex. The model of the atom has changed throughout time. This is because of the creative thought of scientists and the experimental evidence they collected. The diagrams show five different models of atoms suggested by the scientists listed below.
John Dalton, Joseph John Thomson, Ernest Rutherford, Niels Bohr, James Chadwick.
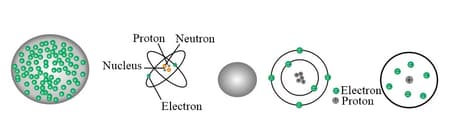
- Identify the model that has similarities with the discoveries of James Chadwick. What did he propose about the atom from the given illustrations?
- Make a list of your observations from the illustrations given about the models given by Rutherford and Niels Bohr.


Important Questions on Structure of the Atom
An element is a substance that cannot be broken down into simpler substances by chemical means. It is composed of atoms that have the same atomic number, that is, each atom has the same number of protons in its nucleus as all other atoms of that element.
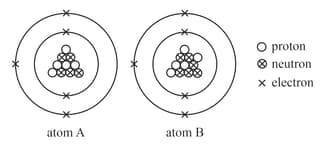
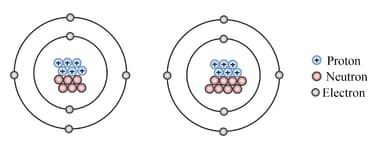
Raju is a th grade student, and his teacher taught him about elements and their subatomic particles. Numerous numerical representations are used while symbolising atoms. His teacher handed him a chart and instructed him to answer some questions based on the given chart. Will you assist Raju in answering the following questions?
- Could you tell which element the atoms represent and write their electronic configuration?
- What do we call such atoms together?
- Write their representation in the form of . Which one is heavier among atoms A and B?
Lithium is a metal that helps in anxiety, and the body's serotonin level works on its response. It is used for medications like anxiety and other mood disorders. It is a silvery-white metal with a symbol .
Lithium being an alkali metal is reactive and has the tendency to lose electrons. That makes it a good conductor. It has been widely used in EV automobiles in the form of Lithium-ion batteries.
On studying the atomic structure of Lithium on the basis of Bohr's postulate, a scientist illustrated a figure as follows.
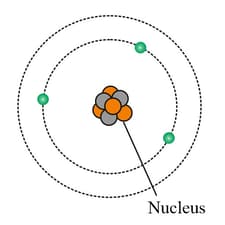
Could you Identify the error made by him and also explain the rings outside the nucleus?
Aarya studied the electronic configurations of some of the elements. He made a graph for the atomic number of the elements and their outer shell electrons. He plotted two different lines on his graph on the basis of the difference in their outer shell electrons. Experimentally he took one element from each of the groups and found that the element from series two shows an exothermic reaction with water while the element from series one does not react at all.
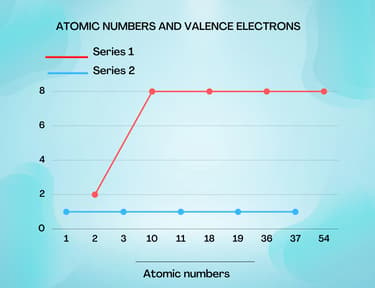
- Series one is not a straight line while series two has. Could you explain what difference it explains?
- What groups of elements do these series represent?
In a classroom, Magnesium, Oxygen, Sodium, Chlorine, and Argon were discussing their properties and uses. Sodium said, "I am very reactive and can catch fire in the air", magnesium said, "I produce white dazzling light when burned", and oxygen said, "I am very important for combustion and the life of the living organisms. Without me, no one can survive on the earth." Chlorine said, "I am the most electronegative element among us and people use me for disinfecting water" but argon stayed quiet and got sad.
Oxygen, sodium, chlorine, and magnesium asked argon, "Why are you sad?" and said, "Now it's your turn. You have to state your characteristics." Then Argon replied, "I am sad because I don't react with any element or compound like you. I am stable and even other elements or chemicals can not combine with me to form new compounds."
After hearing Argon's words, all of them went to the teacher and asked why argon could not be like us. Then the teacher replied that argon has a completely filled outermost shell, so he cannot react with anyone, but other chemical elements can become argon by some chemical means, and to become like argon you first need to know the electronic configuration of the elements.
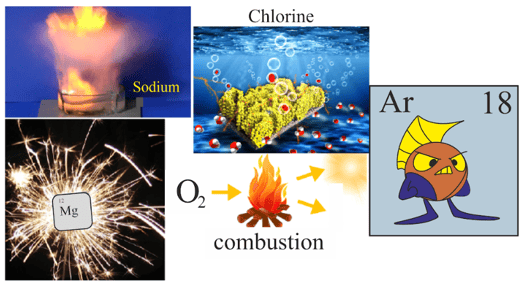
Then these four elements got excited and analysed their electronic configuration, which is given below.
| Element | Atomic number | Electronic configuration |
| Magnesium | ||
| Oxygen | ||
| Sodium | ||
| Chlorine |
Now, by reading the above paragraphs and the table, can you answer the below question?
- Do the elements in the given table have stability? Why?
- If not, how do they attain stability?
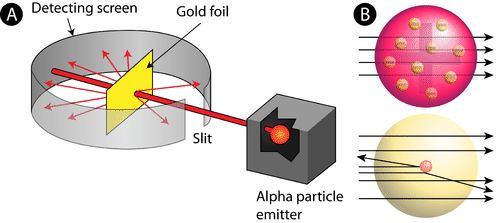
Myra and Anvesh were discussing Rutherford's Gold foil experiment. In the Rutherford Gold Foil experiment, a thin layer of gold is bombarded with minute particles. The gold-foil experiment showed that some of the particles were deflected far away from the center and some of them did not deflect at all. They passed in a straight line. Something dense has been assumed to be at the center of the atom while negatively charged electrons are assumed to be at a great distance from the center.
- What did Rutherford conclude from this experiment? Is the ray diagram given below correct?
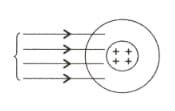
- Myra and Anvesh were asked to draw the complete ray diagram for this experiment?
Three forms of hydrogen are found in the atmosphere- Protium, Deuterium and Tritium, out of which protium is quite abundant, deuterium is said to be formed at the time of the Big Bang and tritium is highly radioactive. Tritium converts into helium through a beta decay.

Every atom when analysed has been found that they have only one orbit and only one electron revolves in that orbit. All of these have one proton in their nucleus as well.
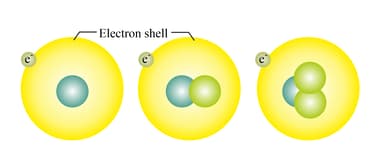
Could you identify the three different species from the picture given? What interference can you arrive at when the mass number and an atomic number of these elements are examined?
In a class, the teacher discussed various features of elements and its atom. An element is a substance that is made entirely from one type of atom. Hence, the students thought that all the atoms of one element always have the same shape, size and mass. But teacher informed them that the atom of the same element can have sometimes different physical properties. The students are very curious about this topic. The teacher decided to explain this topic with some activity and the teacher gave some information about two neutral atoms namely A and B to group and group respectively.
Some information is listed below:
(a) A has electrons, neutrons
(b) B has electrons, neutrons.
Now, help group and group to analyse and answer the following questions:
(i) What are the mass numbers of A and B?
(ii) What is the relation between A and B?
(iii) Which element or elements do these represent?
Rakesh was studying the gold-foil experiment. It showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. The gold foil experiment of Geiger and Marsden paved the way for Rutherford's model of an atom. Rakesh got the problem that of the -particles were found to deflect at angles , If one mole of -particles were bombarded on the gold foil, calculate the number of -particles that would deflect at angles less than .