Aluminium and nitrogen react to form an ionic compound called aluminium nitride. These show the electron arrangement for the two elements:
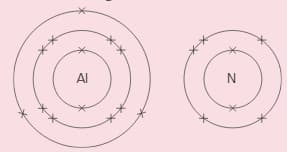
i) Give the electron distribution for the ions formed by the two atoms. ()
ii) What do you notice about these distributions? Explain it.

ii) What do you notice about these distributions? Explain it.

Important Questions on Atoms Combining
- Cotton being woven to make sheets.
Is it a chemical change or a physical change? Give reason.
Aluminium and nitrogen react to form an ionic compound called aluminium nitride.
Name another non-metal that will form an ionic compound with aluminium, in the same way as nitrogen does.
The compound zinc sulphide has a structure like this:
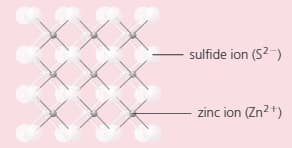
Which does the diagram represent: a giant structure, or a molecular structure?
The compound zinc sulphide has a structure like this:
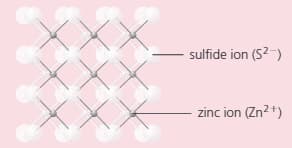
Which type of bonding does zinc sulphide have?
The compound zinc sulphide has a structure like this:
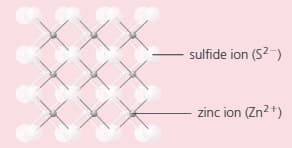
Look carefully at the structure. How many:
i) sulphur ions are joined to each zinc ion?
ii) zinc ions are joined to each sulphur ion?
The compound zinc sulphide has a structure like this:
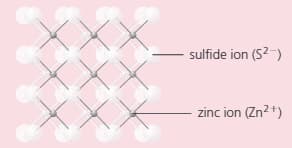
i) Deduce the formula of zinc sulphide.
ii) Is this formula consistent with the charges on the two ions? Explain your answer.
