HARD
Earn 100
Among the following statements, correct statements are:
(i) and have same bond order
(ii) is more stable than
(iii) Bond order of and is same
(iv) MOT explain diamagnetic character of oxygen
(v) HOMO of molecule is
(a)(i), (ii), (v) only
(b)(ii), (iii), (iv) only
(c)(i), (ii), (iii) only
(d)(i) and (ii) only
50% studentsanswered this correctly
Important Questions on Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
The bond lengths (in of and are:
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
How many ions of the following have a bond order of
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
(A) and the other as Reason (R). Examine both the statements carefully and mark the correct choice.
(A) The bond angle in is greater than bond angle in
(R) Due to larger size of , hydrogen bonding does not occur in
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
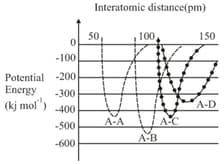
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
What will be the CORRECT order of the C-F bond distance among the following?
and
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.>Structure and Properties of Matter - A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.

